Ionis Reports Promising Trial Results for Donidalorsen in Hereditary Angioedema Patients
Ionis Pharmaceuticals, Inc. reported encouraging outcomes from the Phase 3 OASIS-HAE and OASISplus trials of donidalorsen in individuals with hereditary angioedema, showcasing substantial and lasting declines in average monthly HAE attack rates. Over one year of treatment, both monthly and bi-monthly dosing schedules resulted in more than a 90% improvement in attack rates.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Patients who transitioned to donidalorsen from previous prophylactic treatments experienced an additional 62% reduction in average monthly HAE attack rates from the baseline. Moreover, 84% of patients who made the switch expressed a preference for donidalorsen. Both studies highlighted donidalorsen's positive safety and tolerability profile, even when patients self-administered it using an auto-injector.
These findings will be presented in three late-breaking oral sessions at the 2024 European Academy of Allergy and Clinical Immunology Annual Congress in Valencia, Spain. Additionally, the OASIS-HAE results have been published in The New England Journal of Medicine. Leveraging this data, Ionis is seeking regulatory approval for donidalorsen as a potential HAE treatment.
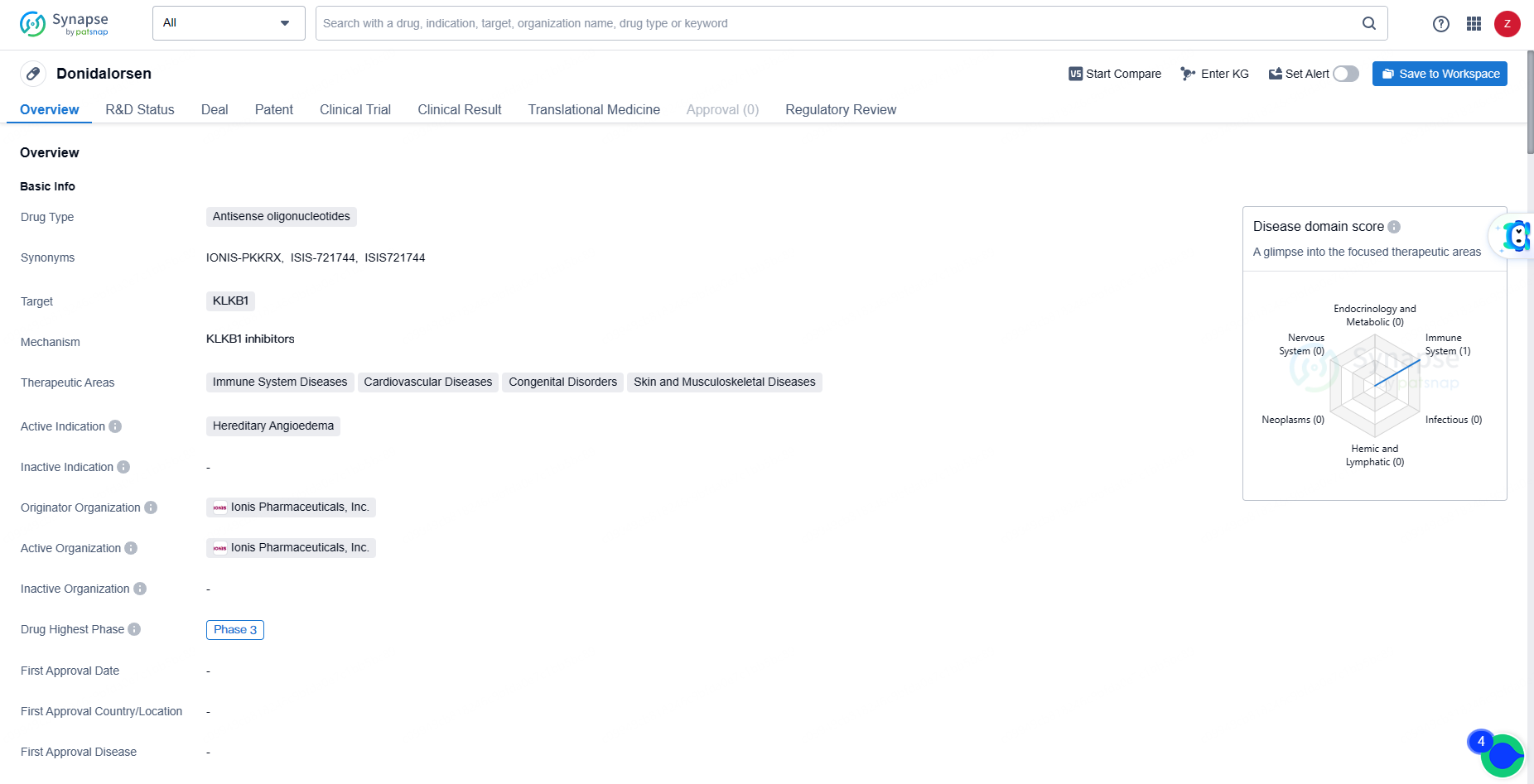
HAE, a rare and possibly fatal genetic disorder, leads to repeated episodes of severe swelling in various body parts such as the hands, feet, genitals, abdomen, face, and throat. Donidalorsen is an investigational RNA-targeted prophylactic therapy designed to decrease the production of prekallikrein, thereby disrupting the cascade that triggers HAE attacks.
"We’re thrilled by the OASIS clinical program results, which we believe set the stage for donidalorsen to revolutionize prophylactic treatment for HAE patients. Despite current therapies, individuals with HAE still endure a significant disease burden, highlighting the need for new prophylactic treatments," commented Brett Monia, Ph.D., CEO of Ionis.
"HAE patients face a lifelong struggle, and I witness the impact daily in my practice. It's crucial for treatments to offer long-lasting efficacy," stated Marc Riedl, M.D., M.S., clinical director, U.S. HAEA Angioedema Center; clinical service chief, Division of Allergy & Immunology, University of California, San Diego. "The OASISplus study demonstrated that patients could transition to donidalorsen without an increased risk of breakthrough HAE attacks, while continuing to improve in quality of life and disease control metrics."
Ionis is planning to submit a New Drug Application to the U.S. Food and Drug Administration this year, moving closer to the launch of our second wholly owned medication. Otsuka Pharmaceutical Co., Ltd., holding the exclusive rights to commercialize donidalorsen in Europe, is also gearing up to submit a Marketing Authorization Application to the European Medicines Agency within the year.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
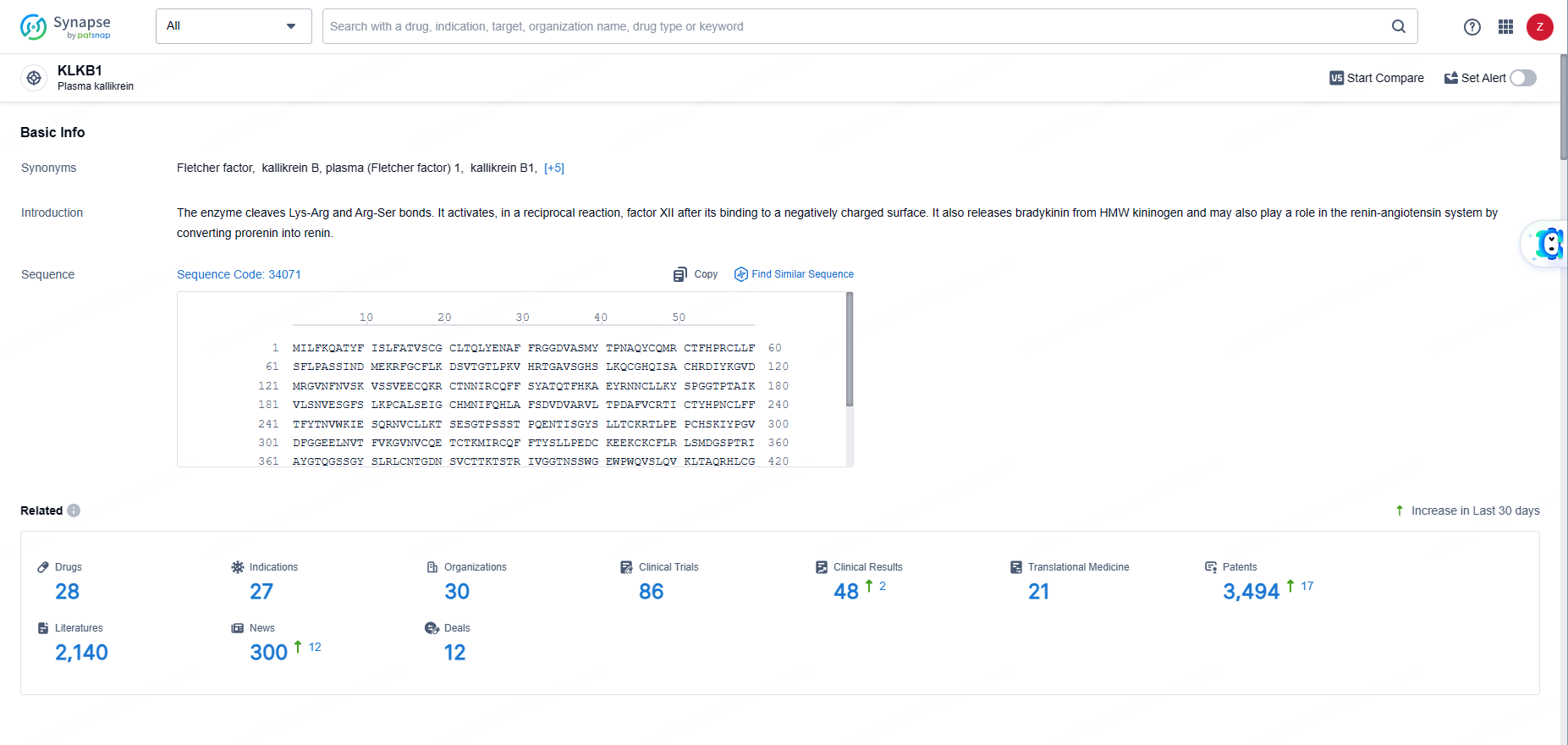
According to the data provided by the Synapse Database, As of June 5, 2024, there are 28 investigational drugs for the KLKB1 targets, including 27 indications, 30 R&D institutions involved, with related clinical trials reaching 86, and as many as 3494 patents.
Donidalorsen is an antisense oligonucleotide drug targeting KLKB1, currently in Phase 3 development for the treatment of Hereditary Angioedema. Its potential therapeutic areas include immune system diseases, cardiovascular diseases, congenital disorders, and skin and musculoskeletal diseases. The Orphan Drug designation further highlights the significance of Donidalorsen in addressing the unmet medical needs of patients with rare genetic disorders.