Is Belumosudil approved by the FDA?
Yes, belumosudil (Rezurock) is FDA approved. The U.S. Food and Drug Administration (FDA) approved belumosudil on July 16, 2021. This approval was granted based on the drug’s efficacy in treating chronic GVHD in patients who have not responded to at least two prior lines of systemic therapy.
Uses and Benefits
Belumosudil is specifically used to manage chronic graft-versus-host disease (GVHD) in both adults and children aged 12 years and older. GVHD is a condition that can occur after an allogeneic stem cell transplant, where the donated bone marrow or peripheral blood stem cells view the recipient’s body as foreign and attack the body.
Administration and Dosage
Belumosudil is available as a 200 mg oral tablet and is typically taken once a day with a meal. It is crucial to follow the prescription instructions carefully:
- Adult and Pediatric Dosage (12 years and older): 200 mg orally once a day until progression of chronic GVHD requiring new systemic therapy.
Patients are advised to take the medication with a full glass of water at the same time each day. The tablets should be swallowed whole and not crushed, chewed, or broken.
Side Effects
Common side effects of belumosudil may include:
- Stomach pain, nausea, diarrhea
- Infections, bleeding
- High blood pressure
- Cough, shortness of breath
- Muscle or bone pain
- Swelling
- Headache
- Feeling weak or tired
Serious side effects that require immediate medical attention include:
- Severe ongoing nausea, vomiting, or diarrhea
- Liver problems (loss of appetite, upper right stomach pain, dark urine, jaundice)
- Signs of infection (fever, chills, sore throat, body aches, unusual tiredness, bruising, bleeding, painful urination, cough with mucus, chest pain, shortness of breath)
Warnings and Precautions
Patients should not use belumosudil if they are allergic to it or have liver or kidney problems. Women of childbearing potential should use effective birth control during treatment and for at least one week after the last dose, as belumosudil can harm an unborn baby. Breastfeeding is not recommended while using this medication and for at least one week after the last dose.
Conclusion
Belumosudil (Rezurock) is an FDA-approved medication effective in managing chronic graft-versus-host disease in patients who have not responded to previous treatments. Patients prescribed this medication should follow their doctor’s instructions carefully and be aware of potential side effects and necessary precautions.
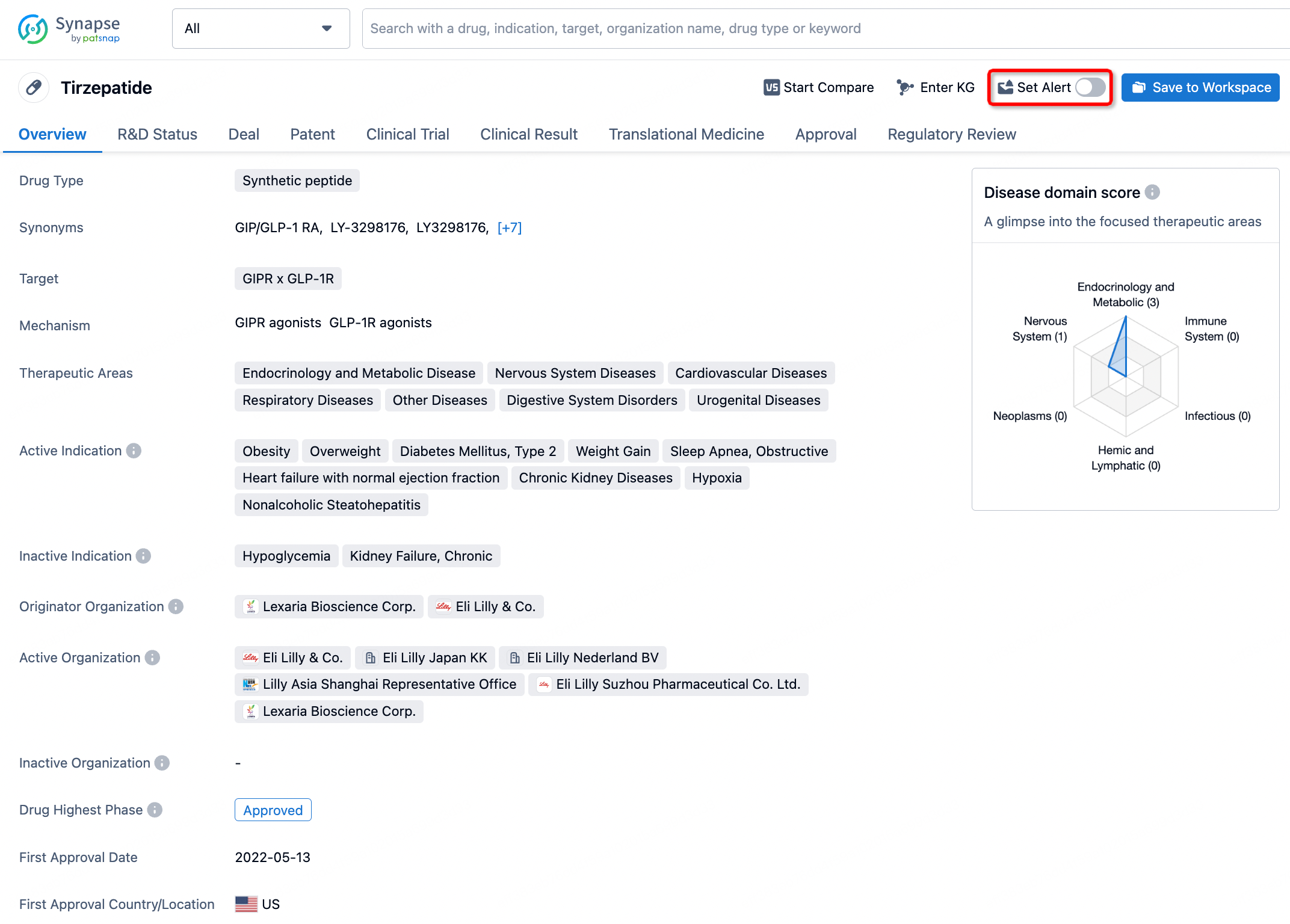
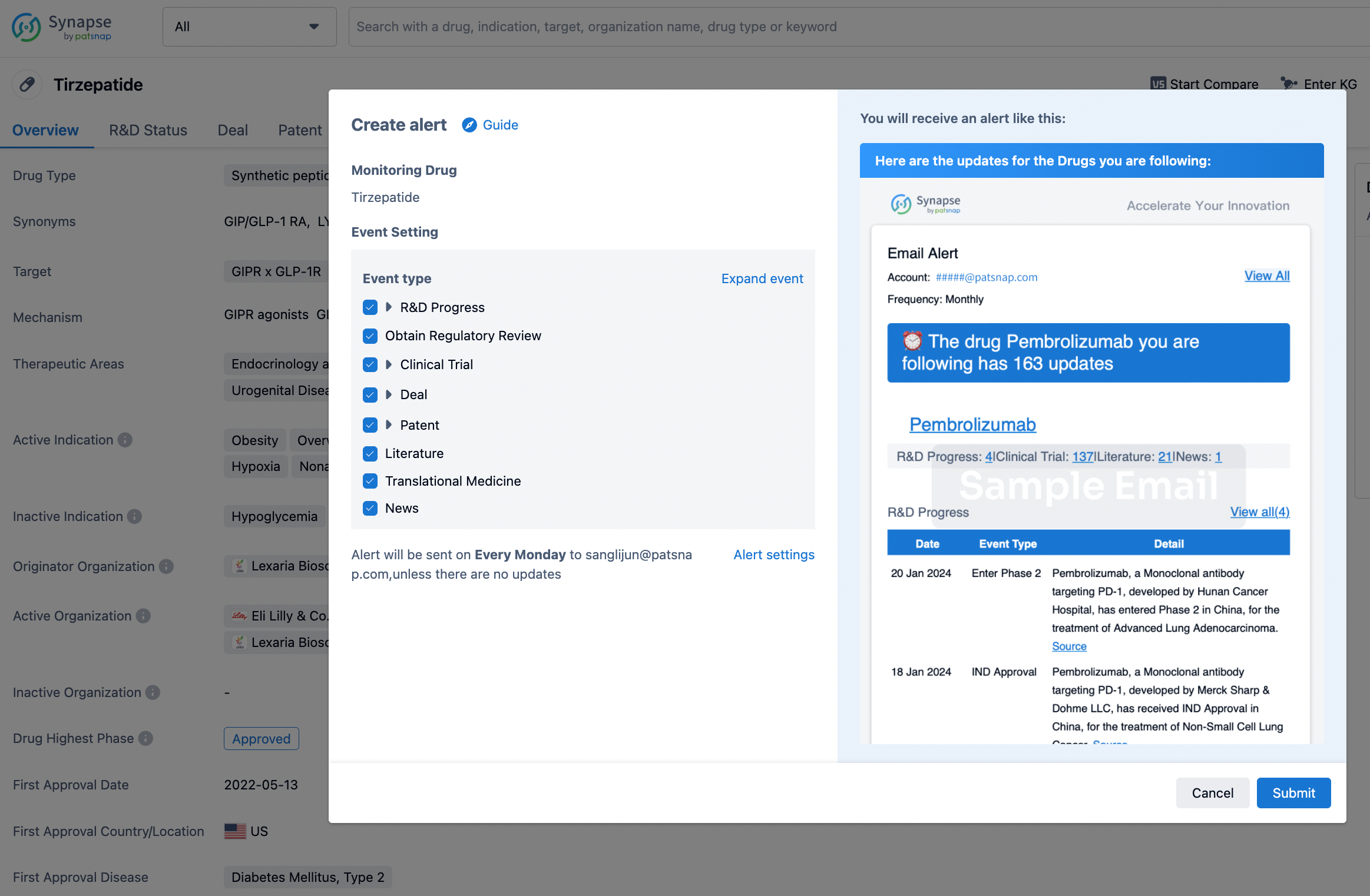
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!