Johnson & Johnson Seeks FDA Approval for SPRAVATO® as Standalone Treatment for Resistant Depression in Adults
Johnson & Johnson disclosed the filing of a supplemental New Drug Application with the U.S. Food and Drug Administration aimed at obtaining approval for SPRAVATO(esketamine) CIII nasal spray to be used as a standalone treatment for adults suffering from treatment-resistant depression.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 Nearly 30% of the approximately 280 million individuals around the globe diagnosed with major depressive disorder suffer from TRD. TRD, or Treatment-Resistant Depression, is characterized by an insufficient response to a minimum of two different oral antidepressants during the same depressive phase.
Nearly 30% of the approximately 280 million individuals around the globe diagnosed with major depressive disorder suffer from TRD. TRD, or Treatment-Resistant Depression, is characterized by an insufficient response to a minimum of two different oral antidepressants during the same depressive phase.
"Many individuals struggling with hard-to-treat depression endure undue periods switching between multiple ineffective treatments, exacerbating their functional and emotional distress, as well as impacting their families," stated Bill Martin, PhD, Global Therapeutic Area Head, Neuroscience, Johnson & Johnson Innovative Medicine.
"We are proud to expand on over a decade’s worth of research that upholds the safety and effectiveness of SPRAVATO. We eagerly anticipate collaborating with the FDA to offer this novel treatment as a monotherapy option for patients," added Bill Martin.
SPRAVATO has obtained FDA approval for use in conjunction with an oral antidepressant to treat adults with TRD and acute suicidal ideation or behavior in adults with MDD. So far, SPRAVATO has been sanctioned in 77 countries and administered to over 100,000 patients globally.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
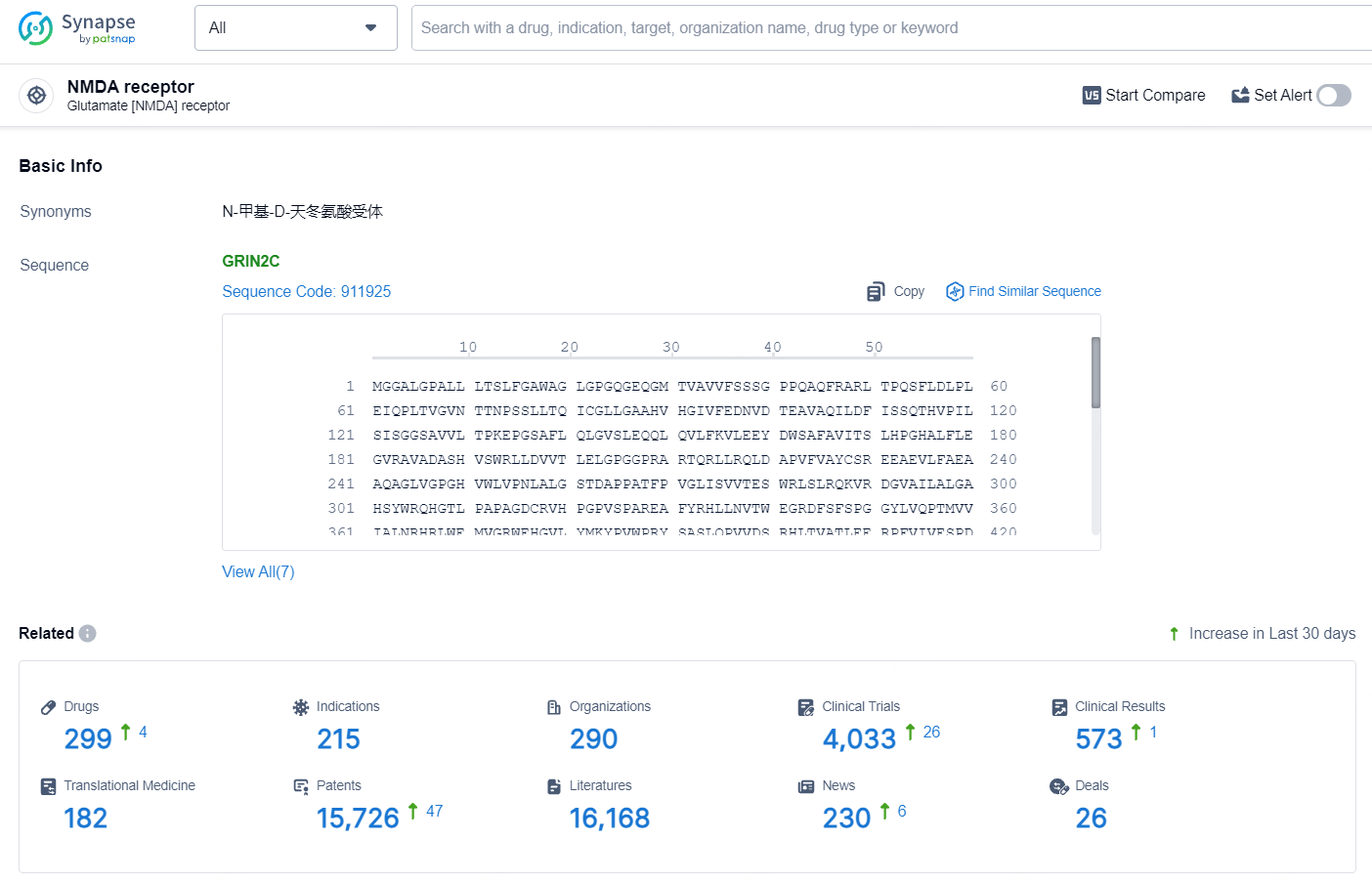
According to the data provided by the Synapse Database, As of July 24, 2024, there are 299 investigational drugs for the NMDA receptor, including 215 indications, 290 R&D institutions involved, with related clinical trials reaching 4033, and as many as 15726 patents.
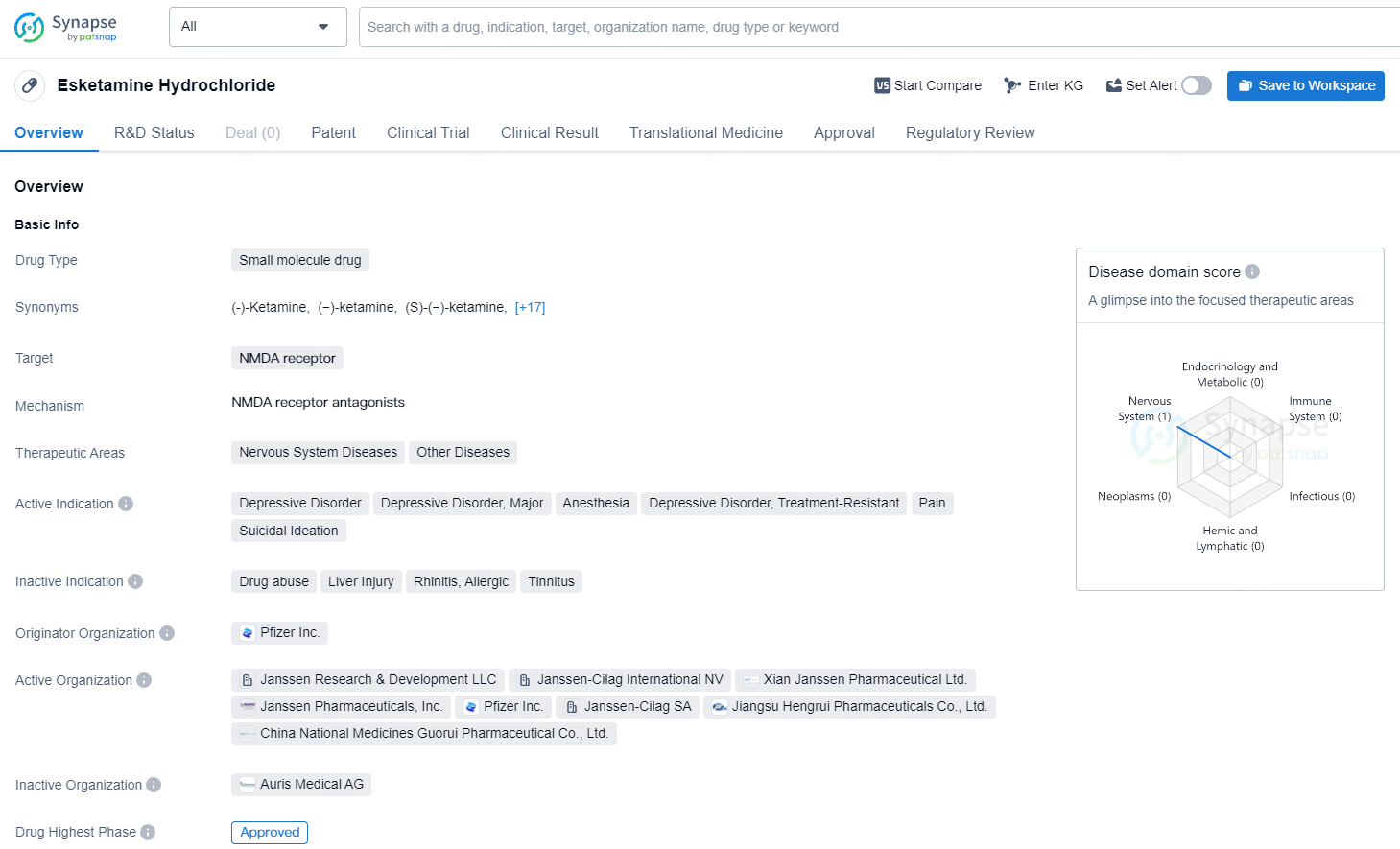
Esketamine Hydrochloride is a small molecule drug that targets the NMDA receptor and has been approved for the treatment of various conditions including depressive disorders, anesthesia, pain, and suicidal ideation. Its first approval was granted in Germany in 1997, and it has received fast-track and breakthrough therapy designations.