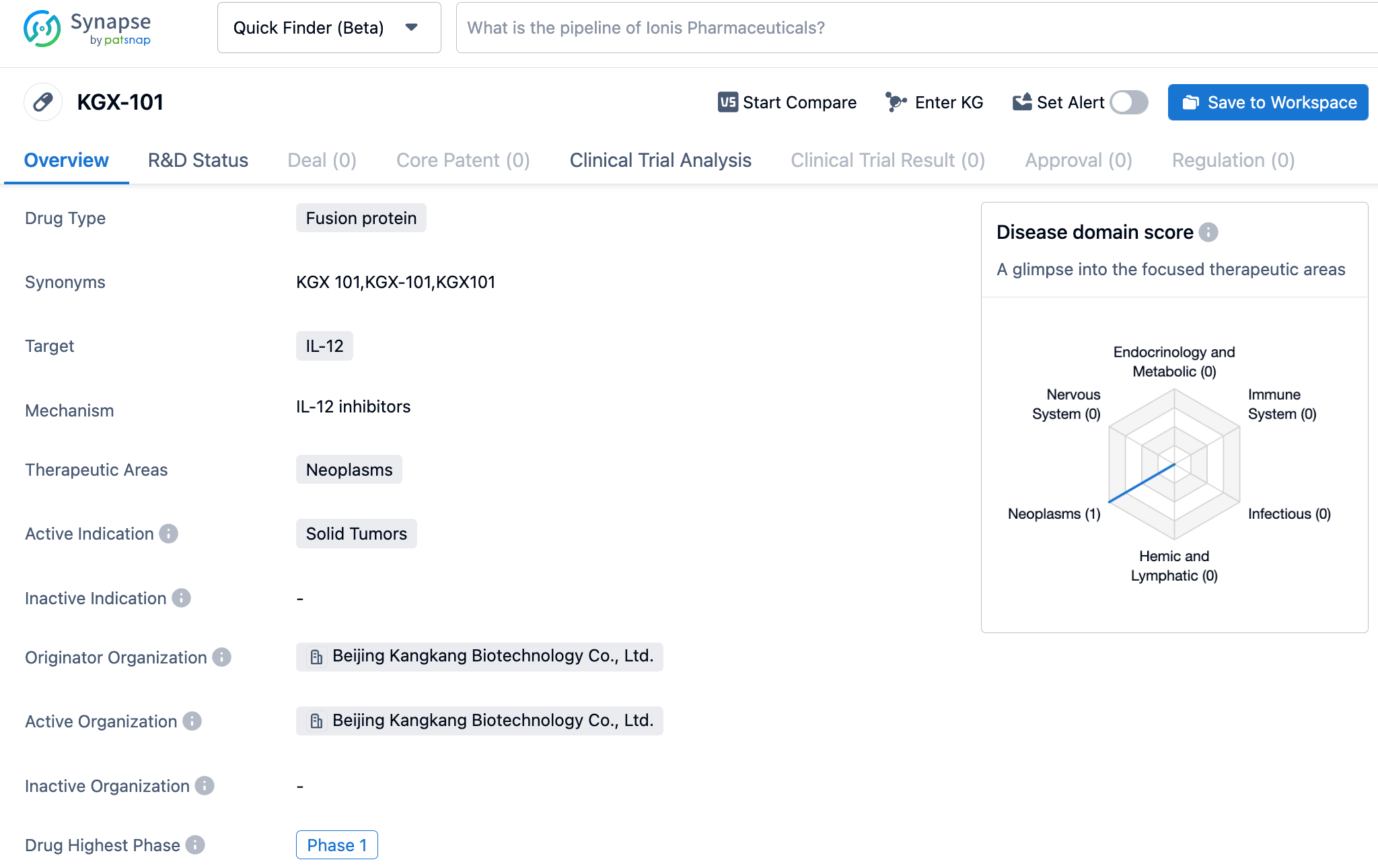
KangaBio's IL-12 prodrug molecule immunotherapy, KGX101, has been approved for clinical use in the United States for the treatment of advanced solid tumors
Recently, KangaBio announced that its independently developed "Tumor-Specific Recombinant IL-12 Fc Fusion Protein for Injection" (pipeline code: KGX101) clinical trial application (IND) has been officially approved by the U.S. FDA. KangaBio will conduct phase 1 clinical trials of KGX101 monotherapy or in combination with anti-PD-L1 antibody treatment for advanced solid tumors in the United States and Australia simultaneously, with several research centers in Australia already begun and the screening of the first patient is currently underway.
KGX101 is a modified and optimized pre-drug molecule of the cytokine IL-12. It can lower the systemic toxicity of cytokines and increase their accumulation in tumor areas by fusing with masking proteins. Meanwhile, by fusing with the Fc region, the pre-drug molecule of KGX101 extends its half-life. Pre-clinical studies showed that KGX101 pre-drug molecule has good anti-tumor efficacy in vivo and reduced systemic toxicity.
Interleukin-12 (IL-12) is a major regulatory factor of innate and adaptive anti-tumor immune responses, composed of a heterodimeric cytokine consisting of IL-12A and IL-12B. IL-12 mainly mediates anti-tumor activity by stimulating T lymphocytes and NK lymphocytes to produce IFN-γ. When tumors are targeted, IL-12 primarily works in the tumor microenvironment (TME) cooperating with other cytokines and immunotherapeutic methods to inhibit tumor angiogenesis. Hence, IL-12 is a powerful ally in immunotherapy combined with checkpoint inhibitors and adoptive T cell transfers.
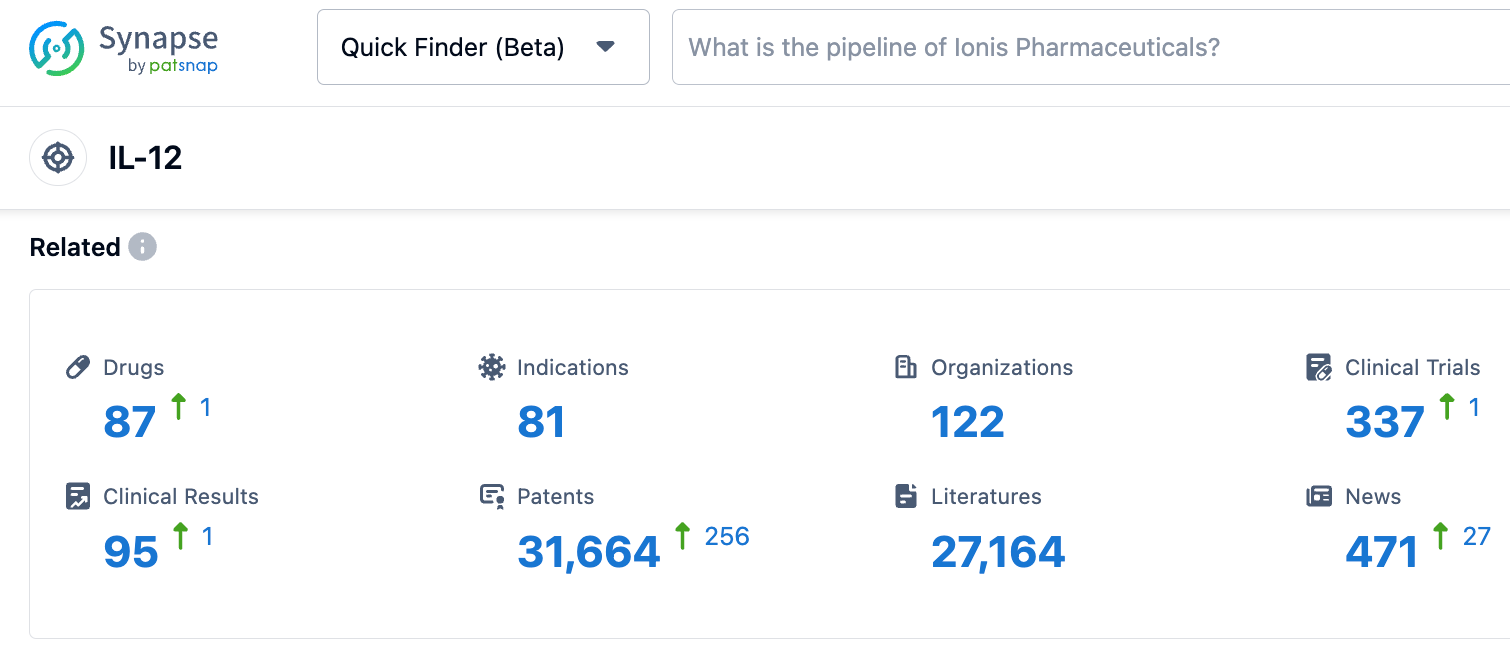
According to the data revealed by the synapse database, as of October 28, 2023, there are 87 drugs under development with IL-12 as the target, covering 81 indications, 122 research institutions involved, relating to 337 clinical trials, and as many as 31634 patents. Interleukin is a large family with various subtypes, each of which has different functions. Some are associated with autoimmune diseases, while others are related to tumor diseases. KangaBio's first product, KGX101, obtained FDA clinical approval, which marks a new milestone for the company's development platform for the next generation of immune-stimulating pre-drug protein molecules. We look forward to the smooth progress of KGX101's subsequent development.