Phase 3 clinical results of Johnson & Johnson's IL-23 inhibitor Guselkumab are positive, significantly relieving symptoms in patients with ulcerative colitis
Recently, Johnson & Johnson announced the results of its Phase 3 clinical trial for its anti-IL-23 antibody Tremfya (guselkumab) in treating moderate to severe ulcerative colitis (UC). The results showed that 77.2% of patients treated with guselkumab reached cumulative clinical remission at week 12 or 24. Relief of symptoms and improvements in patient-reported rectal bleeding and stool frequency were observed just one week after a single intravenous (IV) induction dose. Over two-thirds of patients saw significant symptom relief by week 12.
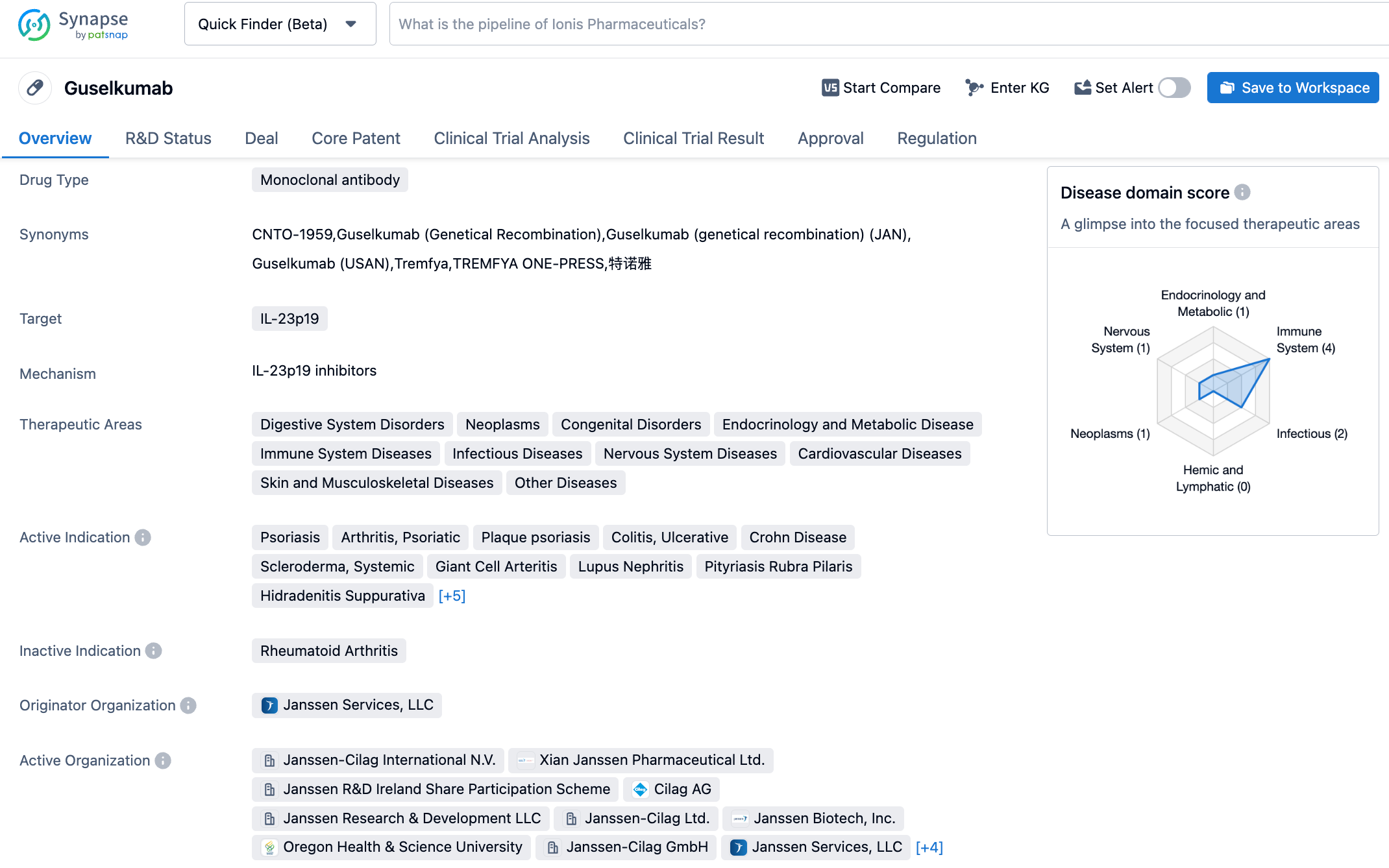
Guselkumab (Tremfya) is a human monoclonal antibody developed by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, targeting the p19 subunit of IL-23. Currently, guselkumab has been approved in the United States, Canada, Japan, and many other countries/regions for the treatment of moderate to severe plaque psoriasis, and psoriatic arthritis.
The QUASAR Phase 3 induction study is a randomized double-blind, placebo-controlled, parallel-group, multicenter study designed to evaluate the efficacy and safety of guselkumab as an induction therapy in patients with moderate to severe active UC, who are inadequately managed by or intolerant to conventional (i.e. thiopurines or corticosteroids) and/or advanced therapies (i.e. TNF-α antagonists, vedolizumab, or tofacitinib). The trial results showed a noticeable improvement in symptoms from week 1 onwards in patients treated with guselkumab compared with placebo, with a sustained increase within 12 weeks. By week 12, 61.5% (259/421) of patients treated with guselkumab achieved clinical remission, significantly higher than the 27.9% (78/280) in the placebo group.
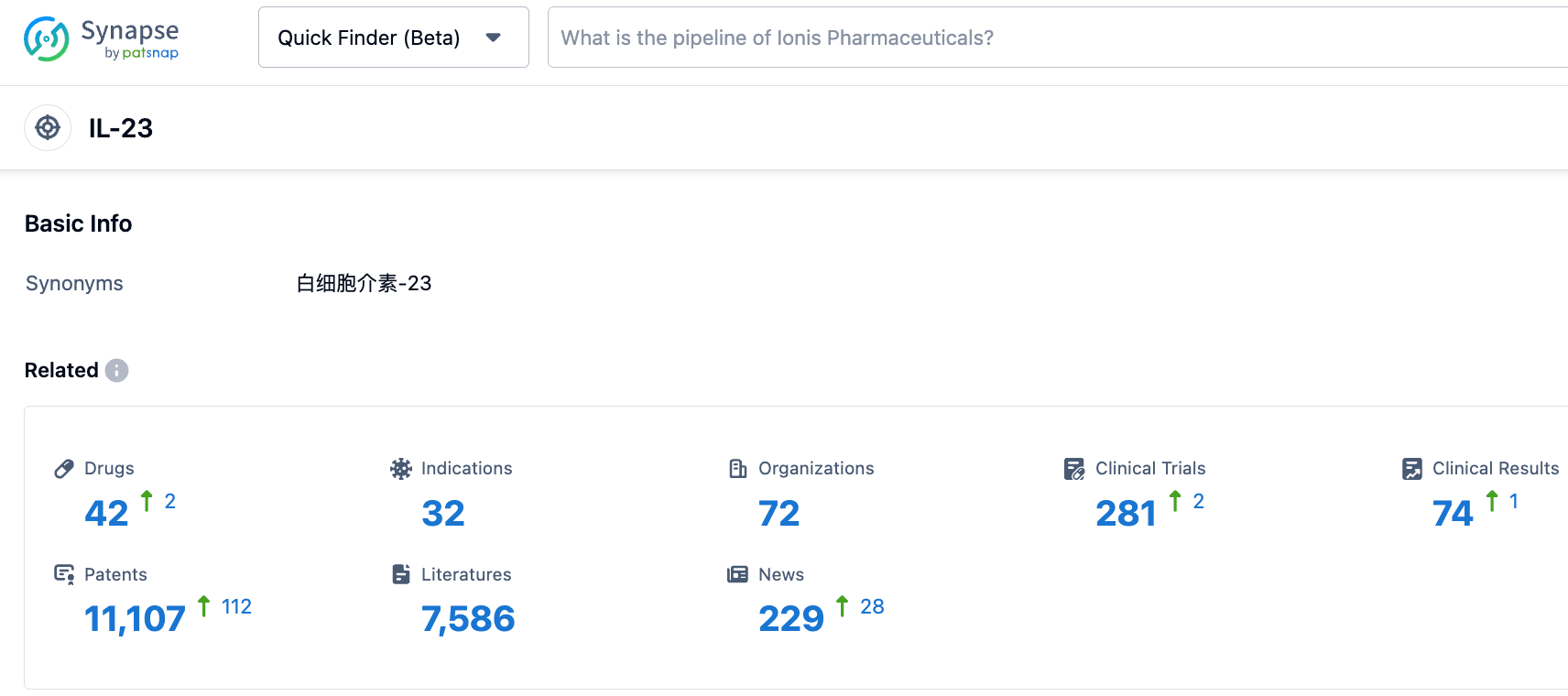
According to the Synapse database, as of October 28, 2023, there are 42 drugs under investigation targeting IL-23, covering 32 indications, 72 research institutions involved, 281 clinical trials conducted, and a whopping 11,102 patents. Interleukins are a large family with various subtypes, each with different functions. Some are associated with autoimmune diseases, while others are linked to cancer. Drugs such as Ustekinumab targeting IL-12/23, Risankizumab and Guselkumab targeting IL-23, Dupilumab targeting IL-4, Secukinumab and Ixekizumab targeting IL-17, and Tocilizumab targeting IL-6, are mainly used to treat autoimmune diseases like plaque psoriasis, psoriatic arthritis, Crohn's disease, ulcerative colitis, and rheumatoid arthritis. It is hoped that Guselkumab will stand out in the competition.