laquinimod: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Laquinimod's R&D Progress
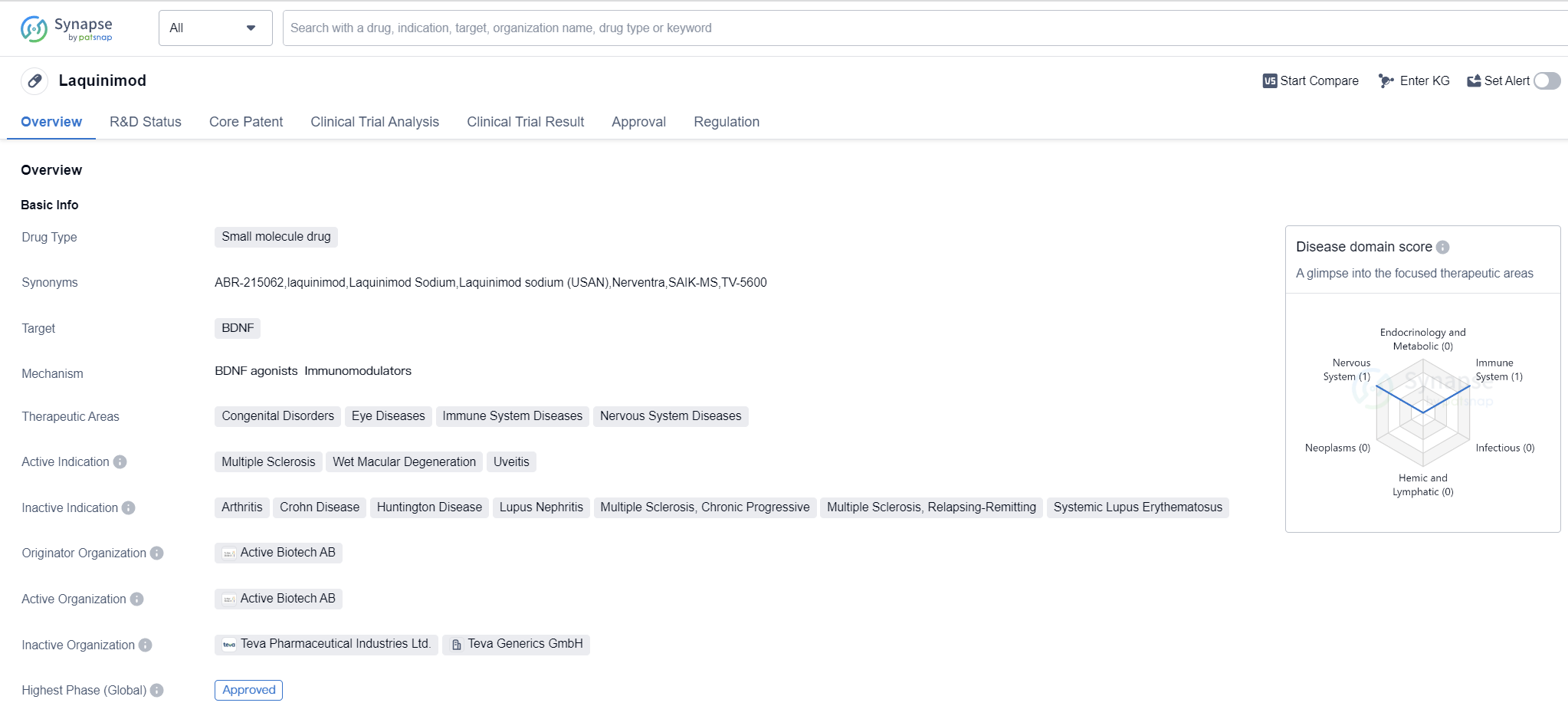
Laquinimod is a small molecule drug that targets the brain-derived neurotrophic factor (BDNF). It is primarily used in the treatment of various therapeutic areas including congenital disorders, eye diseases, immune system diseases, and nervous system diseases. The drug has been approved for the treatment of multiple sclerosis, wet macular degeneration, and uveitis.
Laquinimod was developed by Active Biotech AB, a pharmaceutical company specializing in biomedicine. The highest R&D phase of this drug is approved. It received its first approval in August 2018 in Russia. The approval in Russia suggests that the drug has met the necessary regulatory requirements and has been deemed safe and effective for use in patients.
Laquinimod has been granted orphan drug status, indicating that it is intended to treat rare diseases or conditions that affect a small number of patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for laquinimod: BDNF agonists Immunomodulators
BDNF agonists are substances that mimic or enhance the activity of brain-derived neurotrophic factor (BDNF). BDNF is a protein that plays a crucial role in the growth, development, and maintenance of neurons in the brain. By acting as agonists, these substances activate the BDNF receptors and promote neuronal survival, differentiation, and synaptic plasticity.
From a biomedical perspective, BDNF agonists can be used as potential therapeutic agents for various neurological and psychiatric disorders. For example, they may be explored as a treatment for neurodegenerative diseases like Alzheimer's and Parkinson's, as well as mood disorders such as depression. By enhancing BDNF activity, these agonists aim to support neuronal health and function, potentially improving symptoms and slowing disease progression.
Immunomodulators, on the other hand, are substances that can modify or regulate the immune response. They can either enhance or suppress the immune system's activity, depending on the specific context and desired outcome. Immunomodulators can target various components of the immune system, including immune cells, cytokines, and signaling pathways.
In biomedicine, immunomodulators are of great interest due to their potential therapeutic applications in various diseases. For instance, they can be utilized to boost the immune response against cancer cells, enhancing the effectiveness of immunotherapy. Additionally, immunomodulators may be used to treat autoimmune disorders by suppressing the overactive immune response that targets the body's own tissues.
Overall, BDNF agonists and immunomodulators are two distinct types of substances with different mechanisms of action, but both hold promise for biomedical research and potential clinical applications.
Drug Target R&D Trends for laquinimod
BDNF, or brain-derived neurotrophic factor, is a protein that plays a crucial role in the human body. It is primarily found in the brain and nervous system and is involved in the growth, development, and maintenance of neurons. BDNF promotes the survival of existing neurons and encourages the growth and differentiation of new neurons, thus supporting the overall health and function of the nervous system. It also plays a vital role in synaptic plasticity, which is essential for learning, memory, and cognitive processes. BDNF has been linked to various neurological disorders, and its therapeutic potential is being explored in the pharmaceutical industry.
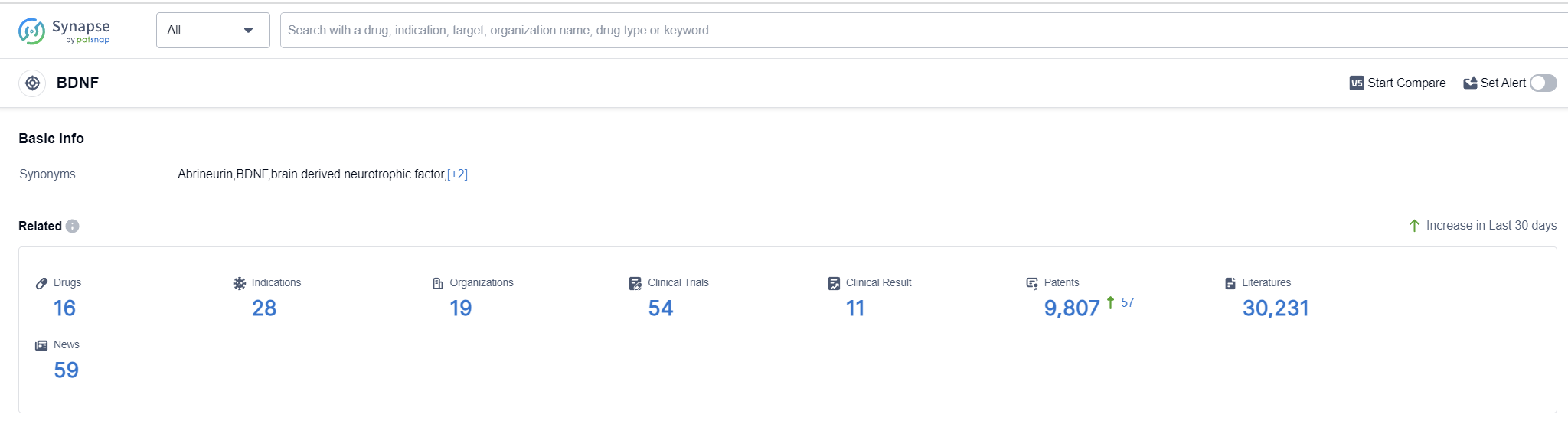
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 16 BDNF drugs worldwide, from 19 organizations, covering 28 indications, and conducting 54 clinical trials.
The analysis of the target BDNF reveals a competitive landscape with multiple companies contributing to its research and development. Active Biotech AB, Tianjin Magpie Pharmaceutical Technology Co., Ltd., and Jinan University are among the companies with the highest stages of development. Approved drugs under the target BDNF have indications such as Multiple Sclerosis, Alzheimer's Disease, and Glaucoma. Small molecule drugs are progressing rapidly, indicating intense competition. China, the European Union, and the United States are actively involved in BDNF research, with China showing progress in Phase 2. Overall, the target BDNF presents opportunities for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
The objective presentation of this information provides a clear overview of the drug Laquinimod and its key characteristics. It is a small molecule drug that targets BDNF and is used in the treatment of various therapeutic areas. The drug has been approved for multiple sclerosis, wet macular degeneration, and uveitis, with its first approval in Russia in 2018. Its originator organization is Active Biotech AB, and it has been granted orphan drug status. This information serves as a foundation for further analysis and evaluation of the drug's potential in the pharmaceutical industry.