The Development and Promise of Suzetrigine in Treating Nervous and Metabolic Disorders
Suzetrigine is a small molecule drug developed by Vertex Pharmaceuticals, Inc. It is designed to target the Nav1.8 channel and is primarily focused on addressing nervous system diseases and endocrinology and metabolic diseases. The drug is being developed to treat various conditions including acute pain, diabetic peripheral neuropathic pain, neuralgia, lumbosacral radiculopathy, and pain.
In terms of regulatory status, Suzetrigine has received priority review, fast track designation, and breakthrough therapy status. These designations are intended to expedite the development and review process for drugs that are considered to have the potential to provide significant benefits over existing treatment options for serious or life-threatening conditions.
As of the latest available information, Suzetrigine has progressed to the New Drug Application (NDA) or Biologics License Application (BLA) stage, which is the highest phase of development on a global scale. This indicates that the drug has undergone extensive preclinical and clinical testing, and the organization is seeking approval for marketing and commercialization from the relevant regulatory authorities.
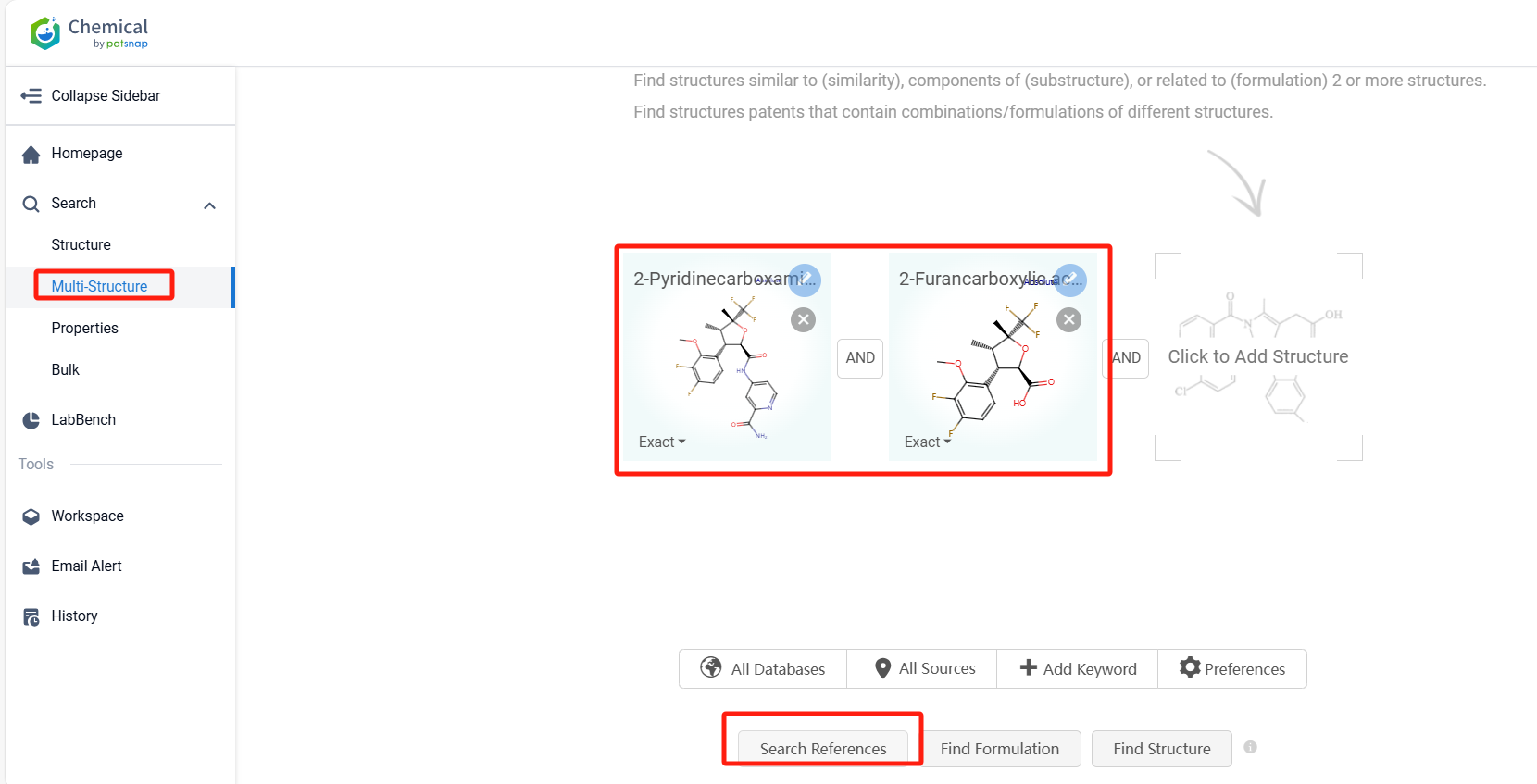
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Suzetrigine. click on search references, and you can query the relevant literature.
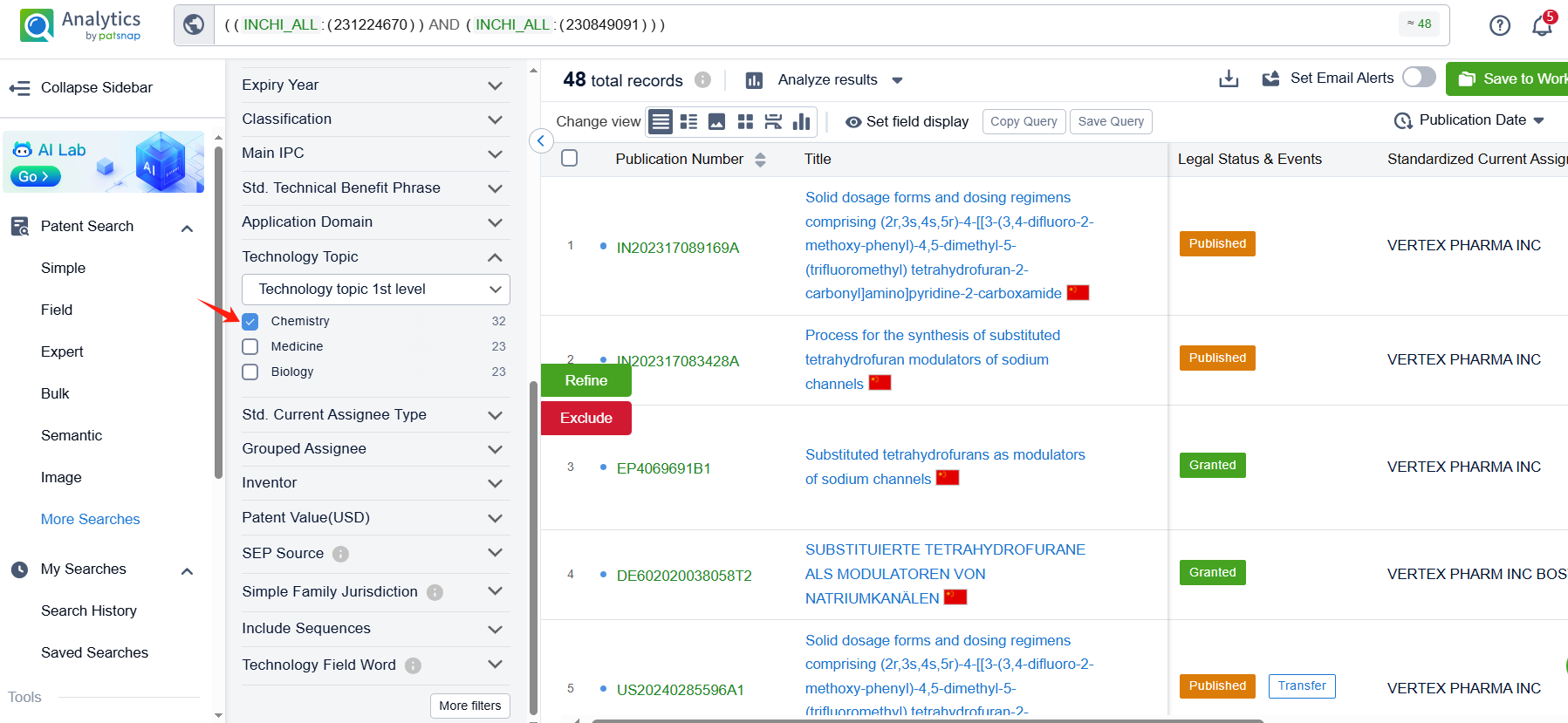
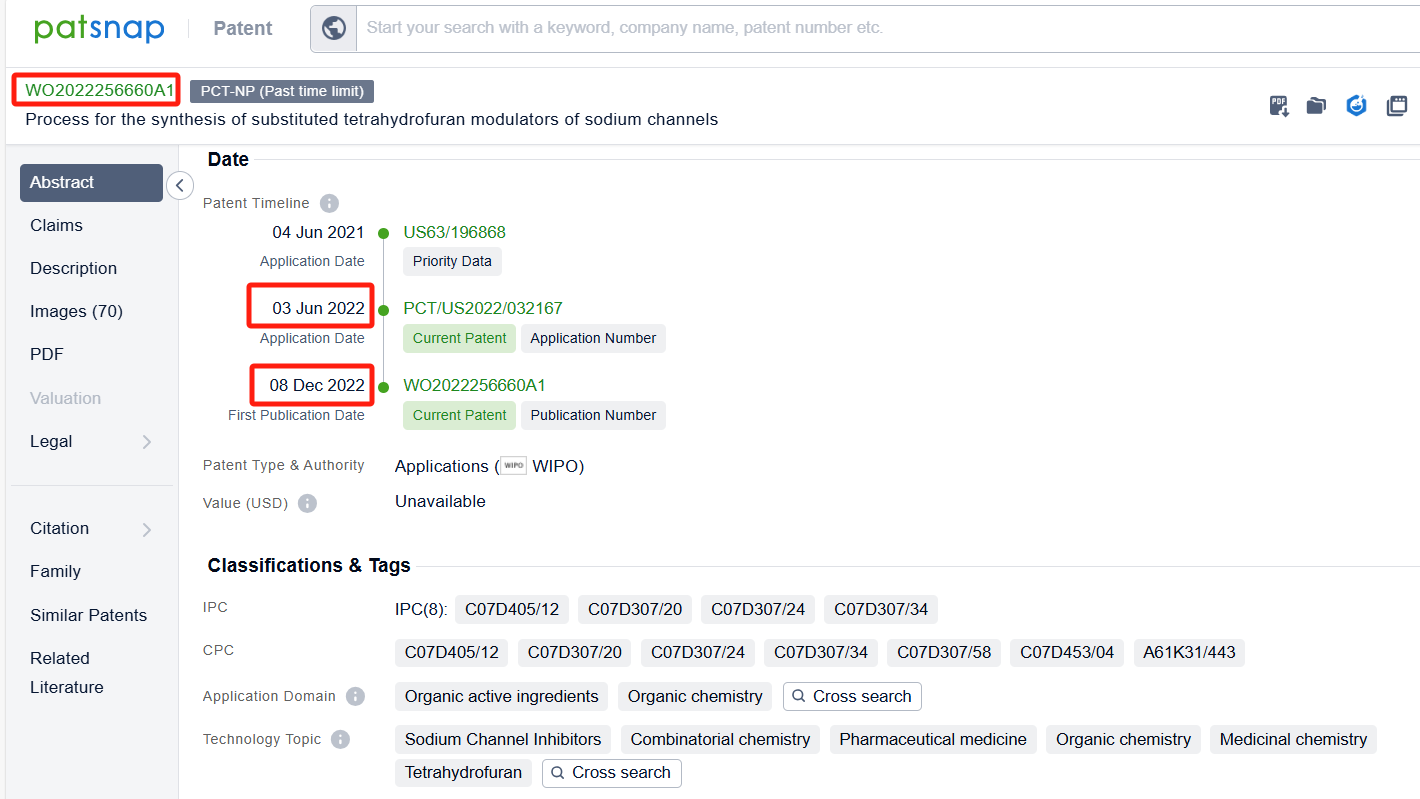
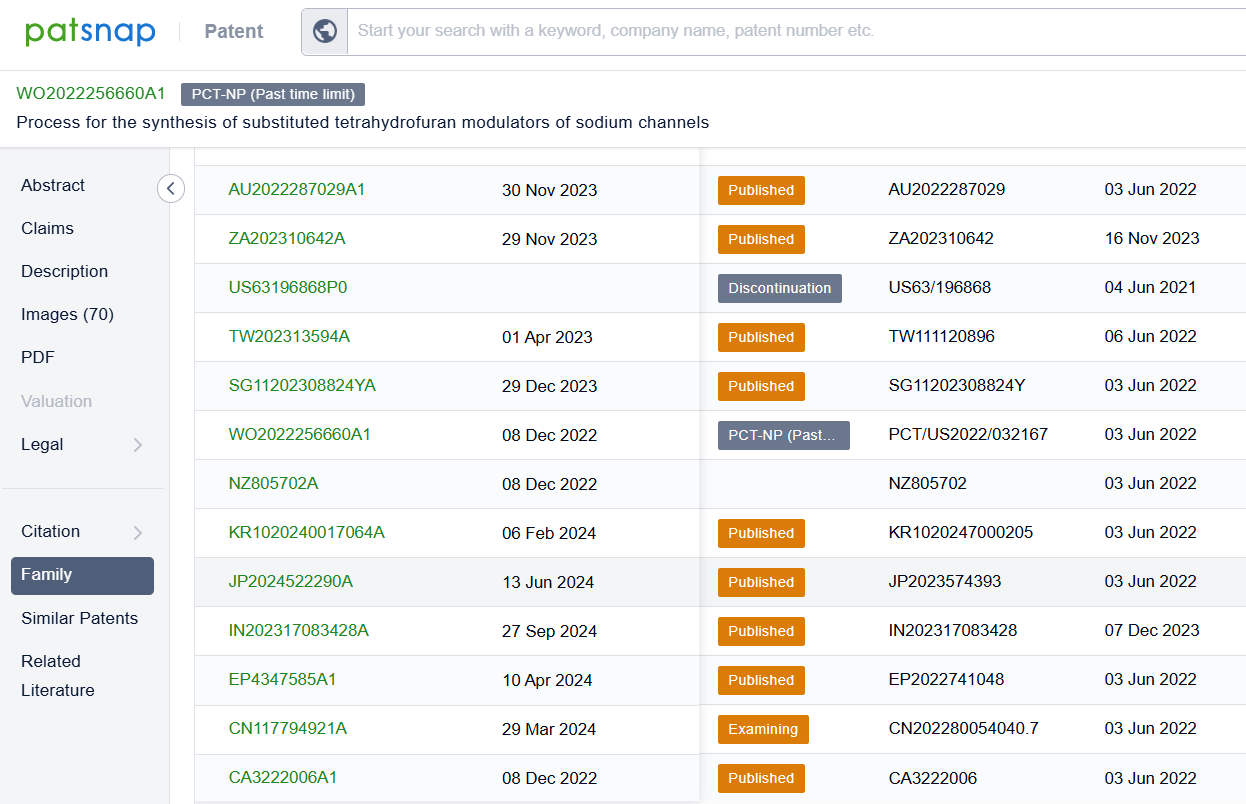
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Suzetrigine process route by Vertex Pharmaceuticals, Inc. and other companies,Such as Vertex Pharmaceuticals, Inc.'s international patent WO2022256660A1 (application date 20220603, publication date 20221208) relates to a method for preparing Suzetrigine,This method has the advantages of high efficiency, simplicity, and economy, and can provide researchers with a reliable method to prepare compounds of Suzetrigine. Its corresponding Chinese patent CN117794921A has also entered substantive examination. By reviewing the aforementioned patent, it can be observed that, to date, no other companies have filed patents regarding improvements to the synthetic routes of Suzettrigine, aside from the original research company.
Overall, the development of Suzetrigine represents a significant advancement in the field of biomedicine, particularly in addressing conditions related to the nervous system and metabolic diseases. The drug's specific targeting of the Nav1.8 channel and its potential to treat various types of pain offer promise for patients who suffer from these debilitating conditions. Furthermore, the regulatory designations it has received underscore its potential to address unmet medical needs and the urgency to bring this therapy to market. However, it is important to note that the information provided is based on the current available data and may be subject to change as the drug continues through the development and regulatory processes.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.