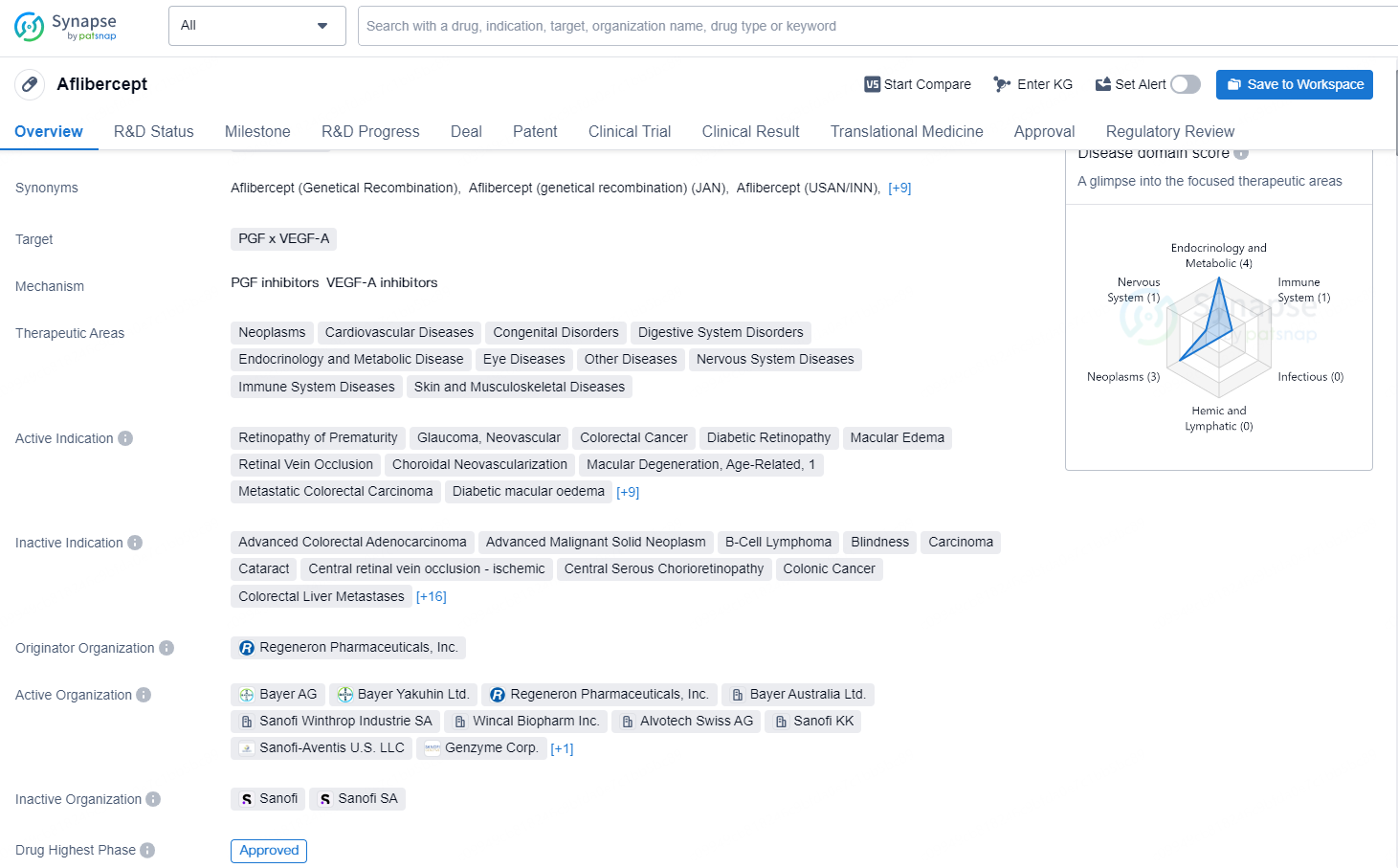
European Commission Approves Sandoz's Biosimilar Afqlir® (Aflibercept)
Sandoz (SIX:SDZ/OTCQX:SDZNY), a worldwide frontrunner in the field of generic and biosimilar drugs, has revealed that the European Commission (EC) has approved the marketing of Afqlir® (aflibercept) 2 mg vial kit and pre-filled syringe for intravitreal administration. This biosimilar is developed as an alternative to the reference product Eylea®. Afqlir® is intended for the treatment of several retinal disorders, including neovascular age-related macular degeneration (nAMD), with the goal of preventing blindness associated with these conditions.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Afqlir® serves as a significant biosimilar asset for Sandoz, and this approval marks a crucial development in the company's growth strategy. The product is projected to be launched in the fourth quarter of 2025.
Claire D’Abreu-Hayling, Sandoz's Chief Scientific Officer, stated, “Vision impairment greatly impacts individuals’ daily lives, affecting everything from employment to social engagements. Access to effective therapies at an early stage is vital for patients striving to preserve and enhance their visual function. The approval of Afqlir® signifies a key advancement in providing an affordable and effective treatment alternative for patients in Europe suffering from diseases like nAMD. This achievement highlights our dedication to enhancing patient outcomes through the availability of high-quality, accessible biosimilars.”
nAMD, a variant of age-related macular degeneration (AMD), is marked by a decline in central vision and is a prominent cause of visual impairment among individuals aged 65 and older. This condition comprises roughly 10 to 20% of all AMD instances but accounts for 90% of severe vision loss associated with AMD. Research indicates that the prevalence of nAMD in countries such as France, Germany, Italy, Spain, the UK, the US, and Japan stands at around 3.6 million individuals, with 2.5 million diagnosed, of whom only 1.7 million are undergoing treatment.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
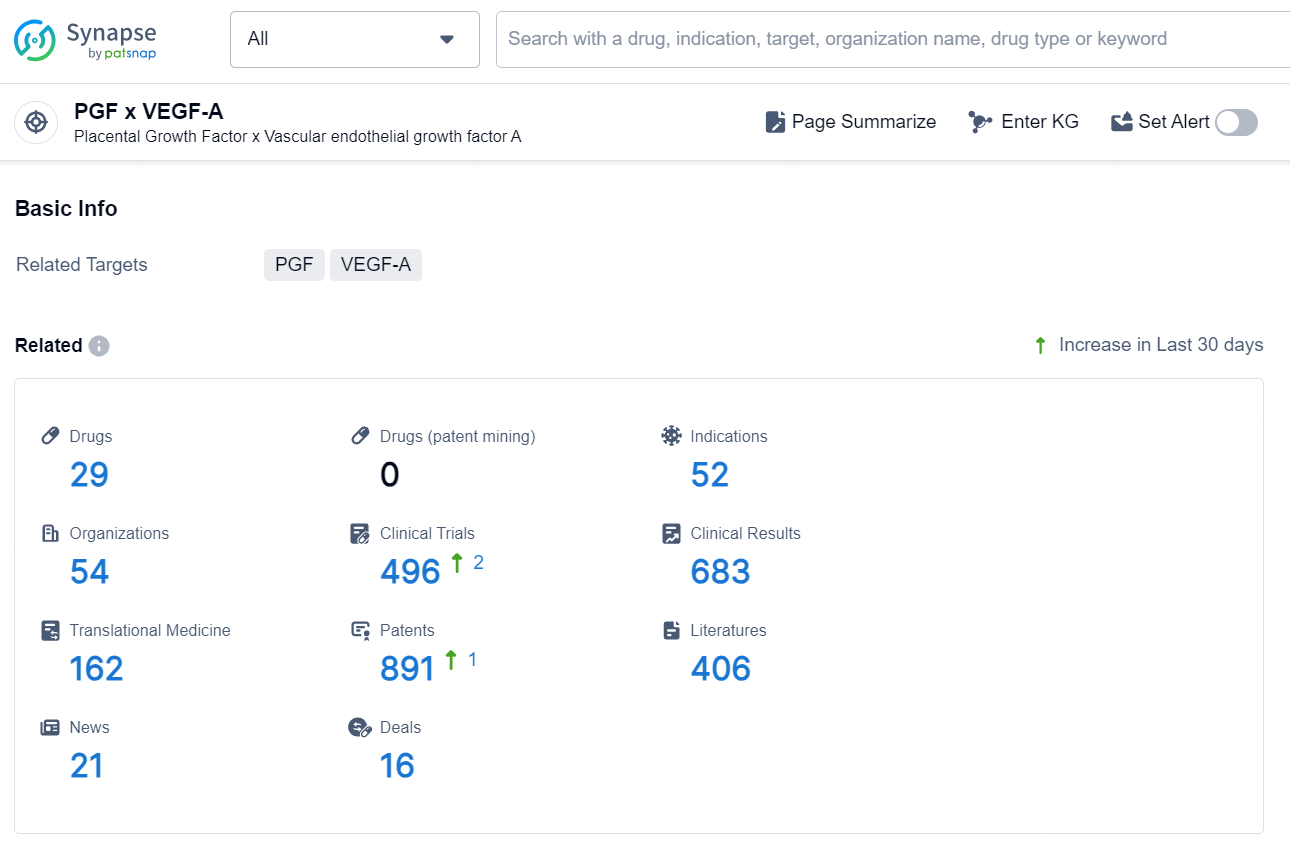
According to the data provided by the Synapse Database, As of November 18, 2024, there are 29 investigational drugs for the PGF x VEGF-A target, including 52 indications, 54 R&D institutions involved, with related clinical trials reaching 496, and as many as 891 patents.
Aflibercept is a fusion protein combining parts of VEGF receptors 1 and 2 with a human IgG1 Fc fragment. It acts as a decoy receptor for VEGF-A, VEGF-B, and placental growth factor (PlGF), preventing them from binding to their natural receptors and halting the pathways that lead to abnormal blood vessel growth and leakage.