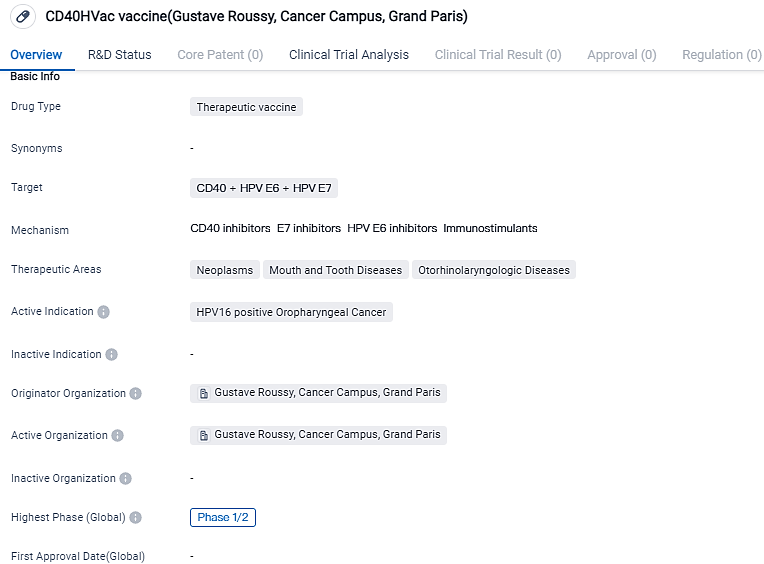
LinKinVax and Gustave Roussy confirmed the initiation of dosage in the Phase I/IIa HPV.DCVax clinical trial for HPV-positive Oropharyngeal Cancer
LinKinVax, a biotech corporation in the clinical stage dedicated to state-of-the-art protein-derived vaccines, and Gustave Roussy, Europe's premier cancer facility and the world's third most respected Cancer Hospital, have reported that Gustave Roussy is treating the initial participant in a Phase I/IIa clinical trial using CD40HVac. This is a fresh therapeutic vaccine candidate for HPV-induced oropharyngeal cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
To assess immune response and safety of CD40HVac coupled with the Poly-ICLC adjuvant (Hiltonol®) in countering cancer-causing HPV in patients suffering from HPV-positive oropharyngeal cancer, we aim to establish the ideal dosage for the Phase II trial, factoring in the potential vaccine candidate’s safety portfolio and its capability to provoke immune reactions.
Several exploratory objectives are also planned to estimate progression-free survival and overall survival. This multi-institutional study is set to explore two dosages of CD40HVac utilising a dose amplification protocol. Up to 24 patients will be recruited in this double-blind, randomised and placebo-controlled study.
Valérie Bouchara, the head of Clinical Operations at LinKinVax, expressed her views on this crucial step in the development of CD40HVac, our cancer therapeutic vaccine: "Our dedicated teams rally around a common goal to deliver significant advancements to patients with scarce therapeutic choices, especially those battling recurrent or metastatic HPV-positive cancers."
According to Dr Caroline Even, who heads the Head and Neck Medical Oncology Unit at Gustave Roussy, “The therapeutic potential of the CD40HVac vaccine offers an exciting adjunct to the current treatment options in patients with HPV-positive oropharyngeal cancer, following the evidence of its safety and effectiveness. We take immense pride in conducting this critical study.”
LinKinVax is focused on developing CD40HVac, a remedial vaccine targeted at HPV-linked malignancies, which operates on a pioneering technology designed to directly target dendritic cells, pivotal elements of the immune system that instigate and manage immune reactions. A recent population-based investigation in the U.S., carried out by the CDC, deduced that HPV 16 and 18 are responsible for 66% of cervical cancers, 55% of vaginal cancers, 79% of anal cancers, and 62% of oropharyngeal cancers.
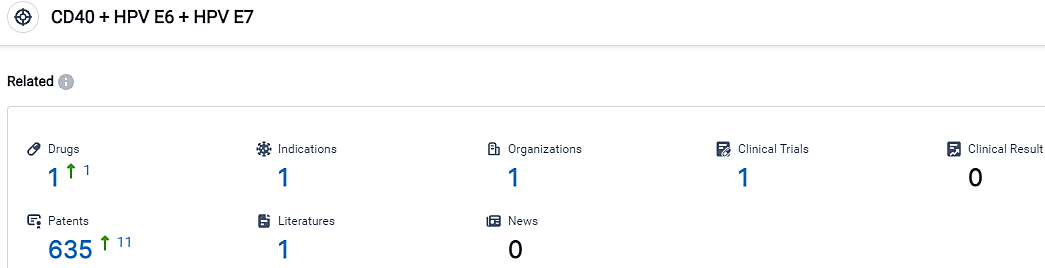
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 22, 2023, there are 1 investigational drugs for the CD40 and HPV E6 and HPV E7 target, including 1 indications,1 R&D institutions involved, with related clinical trials reaching 1,and as many as 635 patents.
Although many HPV-induced tumors can be cured with modern multidisciplinary treatment approaches, it is important to develop new and effective therapeutic vaccines against HPV-associated malignancies to better address the needs of patients.