Successful Phase II Trial of Levicept's Novel Osteoarthritis Treatment LEVI-04
Levicept Ltd, a biotechnology firm dedicated to advancing LEVI-04, an innovative first-of-its-kind therapy for osteoarthritis and various pain conditions, today reports promising outcomes from its Phase II clinical trial. The trial tested LEVI-04, a unique neurotrophin-3 (NT-3) inhibitor, and demonstrated that LEVI-04 is both highly effective and well-tolerated.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase II trial, characterised by multiple arms, multicentre locations, randomisation, double-blind procedures, and a placebo control, included over 510 individuals suffering from knee osteoarthritis-related pain and disability. Conducted across various sites in Europe and Hong Kong, the study was spearheaded by Professor Philip Conaghan MD of the University of Leeds and Leeds Teaching Hospitals NHS Trust, UK.
Standard safety monitoring combined with peripheral nervous system evaluations, indicated that LEVI-04 was well tolerated. There was no observed increase in cases of rapidly progressive osteoarthritis, as determined through meticulous radiographic scrutiny.
In 2020, osteoarthritis affected 595 million people globally, accounting for 7.6% of the worldwide population and representing the most prevalent form of arthritis. The market for OA treatments is projected to be worth over $10 billion.
Kevin Johnson, Chairman of Levicept and Partner at founding investor Medicxi, remarked, “The findings from this extensive Phase II study unequivocally support Levicept’s and its investors’ conviction that LEVI-04 has the potential to revolutionise osteoarthritis treatment. These results significantly bolster Levicept’s position as it considers subsequent strategic steps for advancing LEVI-04 development.”
LEVI-04 is a proprietary p75 neurotrophin receptor fusion protein designed to provide analgesia by inhibiting NT-3 activity and restoring neurotrophin balance. It supplements the endogenous p75NTR binding protein to capture surplus neurotrophins present in chronic pain conditions.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
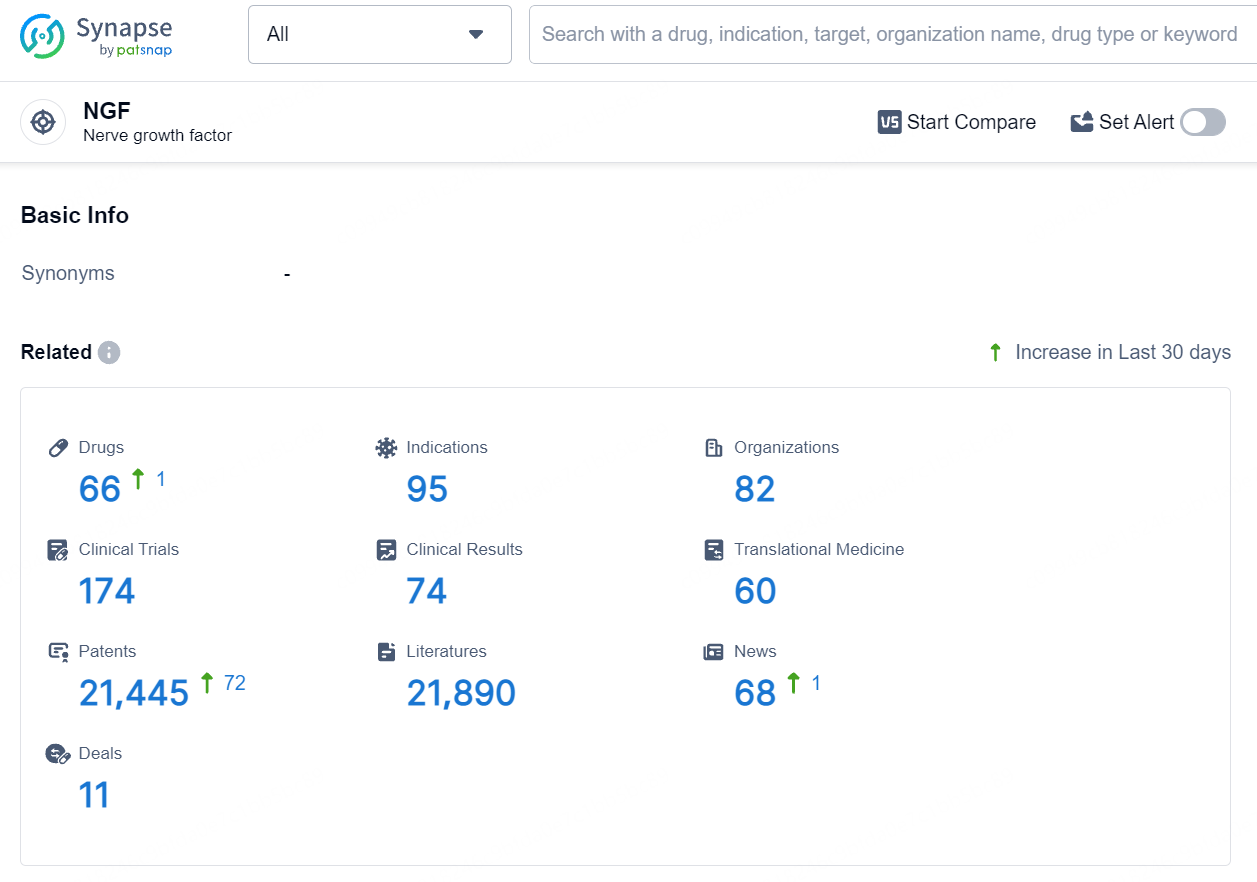
According to the data provided by the Synapse Database, As of August 8, 2024, there are 66 investigational drugs for the NGF target, including 95 indications, 82 R&D institutions involved, with related clinical trials reaching 174, and as many as 21445 patents.
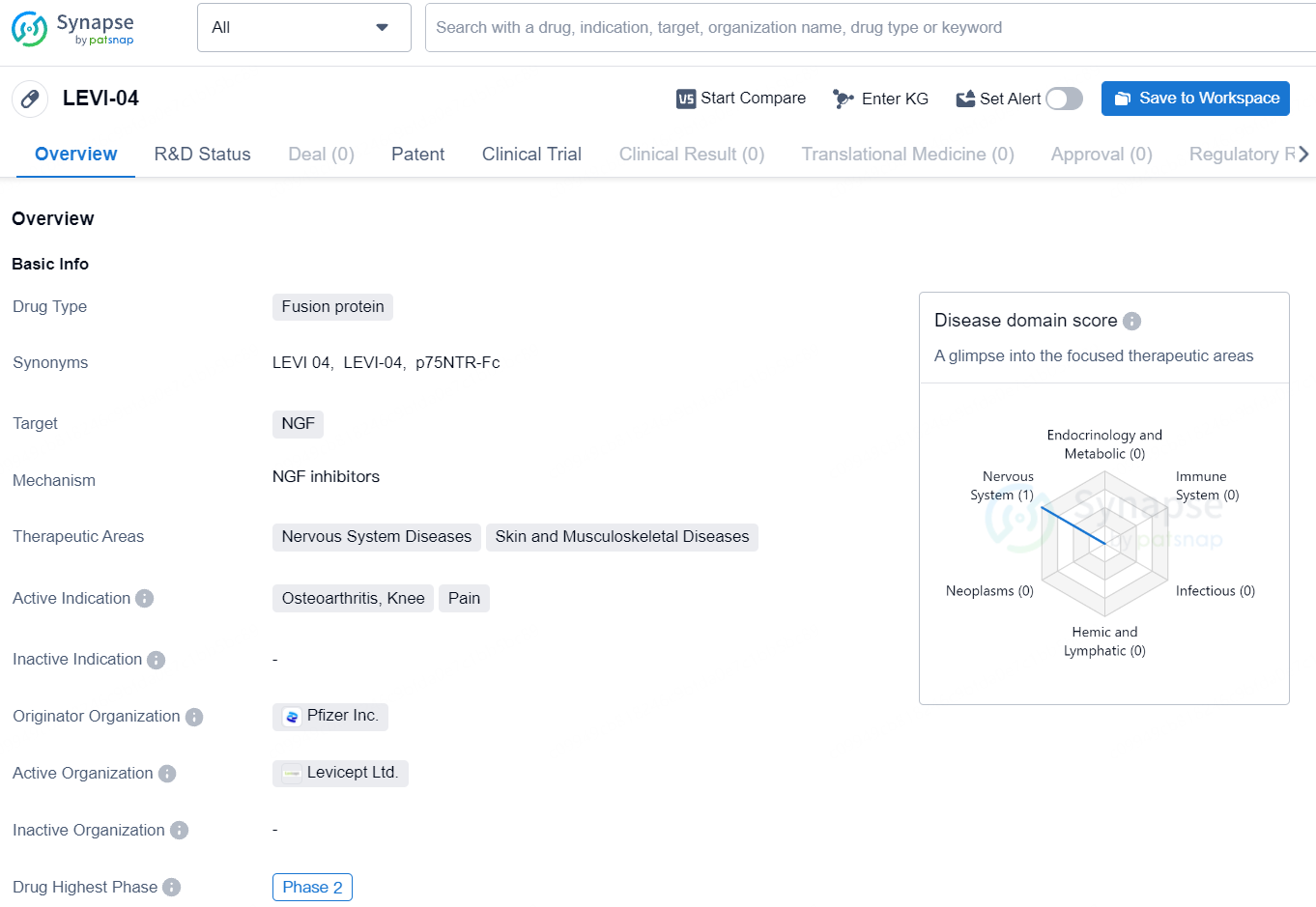
LEVI-04 presents significant potential as a fusion protein drug targeting NGF for the treatment of nervous system diseases, skin diseases, and musculoskeletal diseases, with a specific active indication for osteoarthritis of the knee and pain management. With its current status in Phase 2 of development and the involvement of Pfizer Inc. as the originator organization, LEVI-04 represents a promising candidate in the pharmaceutical industry's efforts to address critical medical needs.