MoonLake Immunotherapeutics Initiates Phase 3 of IZAR Program for Sonelokimab Nanobody® in Psoriatic Arthritis Patients
MoonLake Immunotherapeutics, a biotechnology firm in the clinical stage dedicated to developing advanced treatments for inflammatory conditions, has reported that the initial patients have undergone screening at trial locations in the United States as part of its international Phase 3 clinical trial program, IZAR. This program is assessing sonelokimab, a novel investigational Nanobody® intended for the treatment of inflammatory diseases, in individuals suffering from active psoriatic arthritis (PsA).
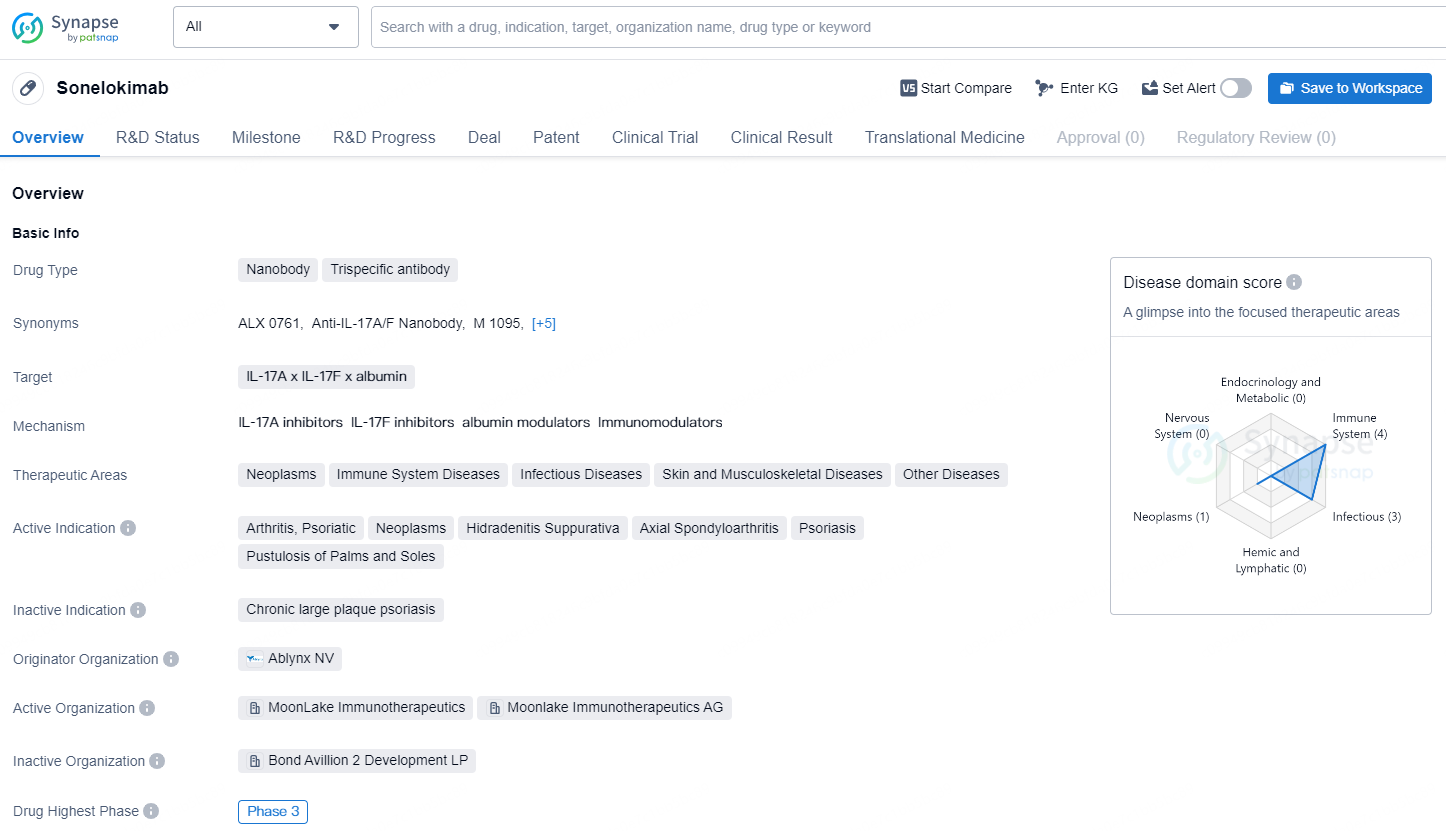
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Psoriatic arthritis (PsA) is a long-lasting, debilitating inflammatory disease that impacts not only peripheral joints and skin but also affects other areas, including entheses, nails, and axial joints. This condition, which spans multiple domains, has significant unmet needs, particularly in its management of inflammation and pain across these various areas at the same time. While the precise mechanisms contributing to PsA remain partially understood, research suggests that the activation of the IL-17 pathway is a key factor in its pathophysiology. Sonelokimab, a Nanobody®, is engineered to directly address inflammation by inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers. Its reduced size as a Nanobody® enhances its ability to penetrate and target hard-to-reach inflamed tissues more effectively than traditional antibodies.
Building on the encouraging outcomes of the Phase 2 ARGO trial, the Phase 3 IZAR program plans to recruit around 1,500 adult participants across two studies: IZAR-1 (NCT06641076) and IZAR-2 (NCT06641089). These global, randomized, double-blind, placebo-controlled trials aim to assess the efficacy and safety of sonelokimab over a 52-week period. IZAR-1 will focus on patients who have not previously received biologic treatments and will include an assessment of radiographic progression, while IZAR-2 will enroll patients with an insufficient response to TNF-α inhibitors and will uniquely include risankizumab, a monoclonal antibody that targets IL-23, as an active reference. The primary endpoint, measured by the American College of Rheumatology (ACR) 50 response compared to placebo, alongside critical secondary endpoints for both trials, is expected to be evaluated by Week 16. The IZAR program will investigate doses of sonelokimab at 60mg and 120mg, with primary endpoint results for both studies anticipated in the first half of 2026.
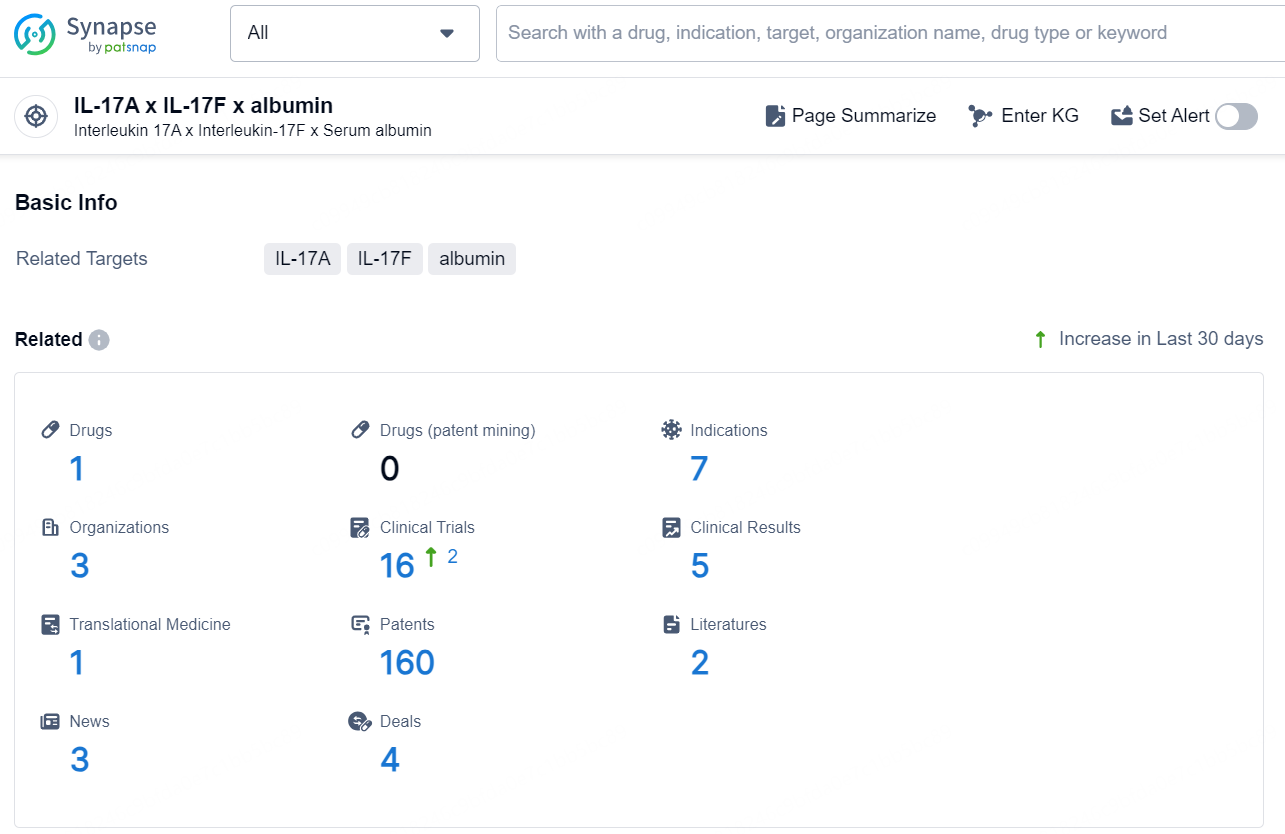
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 1 investigational drug for the IL-17A, IL-17F target, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 16, and as many as 160 patents.
Sonelokimab is a nanobody and trispecific antibody drug that targets IL-17A, IL-17F, and albumin. It is being developed for the treatment of various therapeutic areas including neoplasms, immune system diseases, infectious diseases, skin and musculoskeletal diseases, and other diseases. The active indications for Sonelokimab include arthritis, psoriatic, neoplasms, hidradenitis suppurativa, axial spondyloarthritis, psoriasis, and pustulosis of palms and soles.