Nalidixic Acid Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Nalidixic Acid's R&D Progress
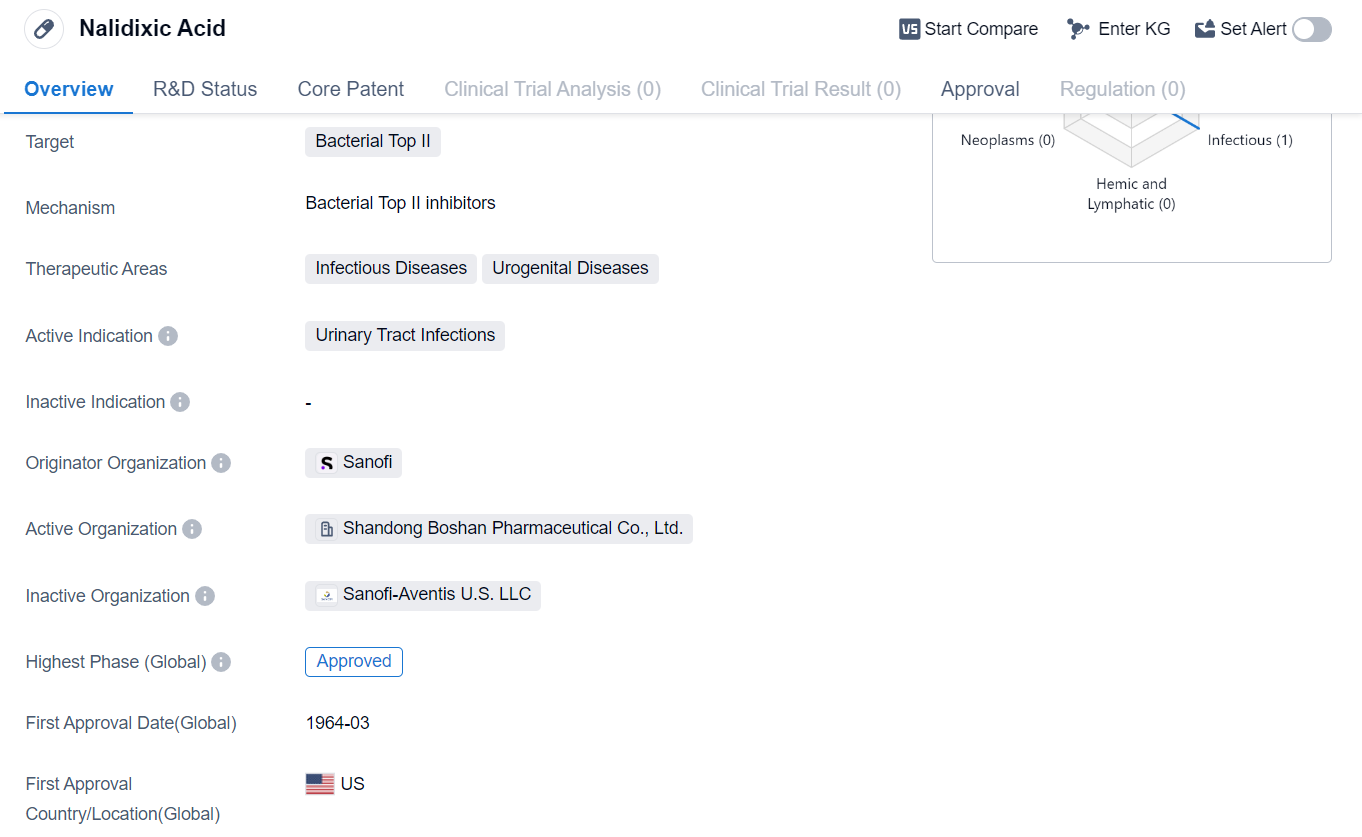
Nalidixic Acid is a small molecule drug that falls under the category of biomedicine. It primarily targets bacterial Top II, making it effective against various infectious diseases and urogenital diseases. The drug has been specifically approved for the treatment of urinary tract infections.
The originator organization of Nalidixic Acid is Sanofi, a renowned pharmaceutical company. The drug has successfully completed the highest phase of clinical trials and has received approval globally. The global approval for Nalidixic Acid was granted in March 1964, with the United States being the first country to approve its use.
As a small molecule drug, Nalidixic Acid works by inhibiting the activity of bacterial Top II, which is crucial for the replication and repair of bacterial DNA. By targeting this specific enzyme, the drug effectively disrupts the growth and survival of bacteria, particularly those causing urinary tract infections.
Urinary tract infections are a common type of infection that affects the urinary system, including the bladder, kidneys, and urethra. These infections are primarily caused by bacteria, and Nalidixic Acid has proven to be an effective treatment option for such cases.
The approval of Nalidixic Acid in global markets highlights its significance in combating urinary tract infections. The drug's long history since its first approval in 1964 demonstrates its established position in the pharmaceutical industry.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nalidixic Acid: Bacterial TopⅡinhibitor
Bacterial Top II inhibitors are a type of drugs that specifically target and inhibit the activity of bacterial topoisomerase II enzymes. Topoisomerase II is an essential enzyme involved in DNA replication and repair in bacteria. By inhibiting this enzyme, bacterial Top II inhibitors disrupt the normal DNA processes in bacteria, leading to DNA damage and ultimately bacterial cell death.
From a biomedical perspective, bacterial Top II inhibitors are considered as potential antibiotics. They selectively target bacterial topoisomerase II enzymes, which are distinct from the human counterpart, thus minimizing the risk of adverse effects on human cells. By specifically targeting bacterial DNA replication and repair, these inhibitors can effectively kill bacteria and treat various bacterial infections.
It's important to note that bacterial Top II inhibitors should be used under medical supervision and prescription, as their misuse or overuse can lead to the development of antibiotic resistance or adverse effects.
Drug Target R&D Trends for Nalidixic Acid
Based on the analysis of the provided data, the current competitive landscape of target Bacterial Top II shows that several companies are actively involved in the research and development of drugs targeting this target. Daiichi Sankyo Co., Ltd., Santen Pharmaceutical Co., Ltd., Ceolia Pharma Co., Ltd., CHIESI Farmaceutici SpA, Grand Pharmaceutical Group Limited, Chuchang Investment Group Co. Ltd., SuZhou Stainwei Biotech Inc., Taisho Pharmaceutical Holdings Co., Ltd. have made significant progress with drugs in the approved phase.
The indications for the approved drugs under the target Bacterial Top II cover a wide range of bacterial infections and related conditions. This indicates the potential for these drugs to address various medical needs.
The development of small molecule drugs is progressing rapidly under the current target, indicating a focus on this drug type. However, there is no mention of biosimilars in the provided data, suggesting that competition around the innovative drug may not be intense.
China is showing progress in the development of drugs targeting Bacterial Top II, with several drugs in the approved and inactive phases. This highlights the growing presence of China in the pharmaceutical industry.
Overall, the current competitive landscape of target Bacterial Top II is dynamic, with multiple companies and countries actively involved in research and development. The future development of this target holds potential for addressing various bacterial infections and related indications.
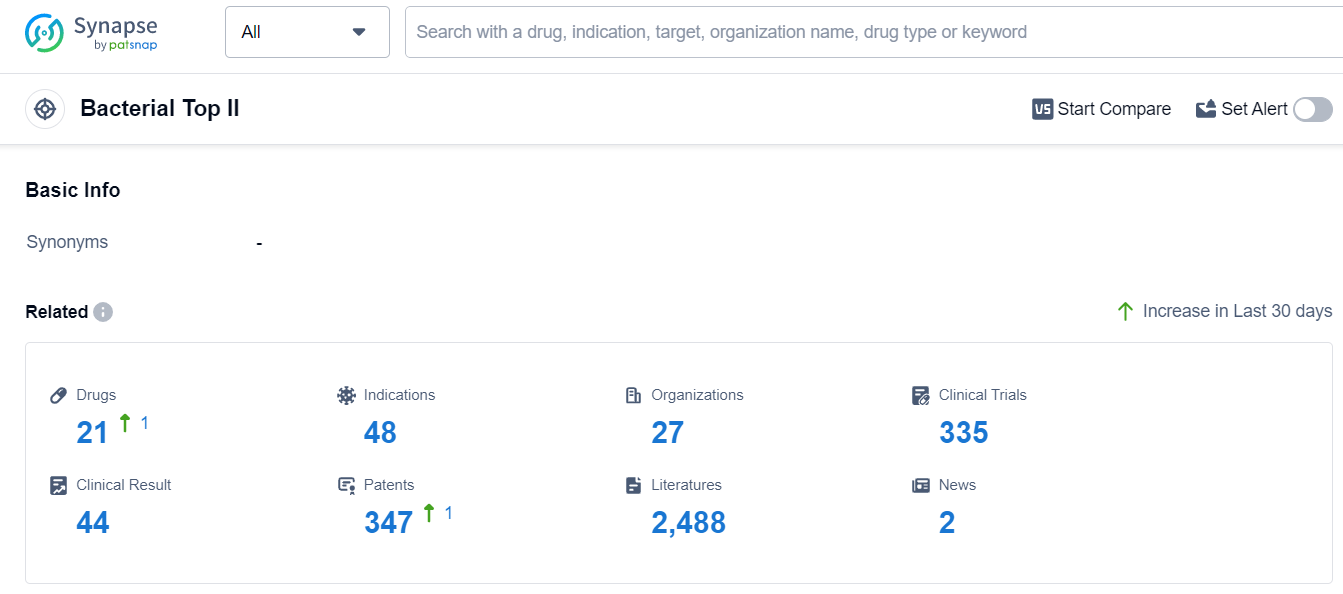
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 21 Bacterial Top II drugs worldwide, from 27 organizations, covering 48 indications, and conducting 335 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nalidixic Acid is a small molecule drug developed by Sanofi that targets bacterial Top II. It has been approved for the treatment of urinary tract infections, making it a valuable therapeutic option for patients suffering from this condition. Its approval in global markets further emphasizes its effectiveness and importance in the field of biomedicine.