Nippon Kayaku and Celltrion declared the Japanese market's endorsement of Adalimumab Biosimilar Monoclonal Antibody
Nippon Kayaku Co., Ltd. in collaboration with Celltrion Healthcare Japan K.K. has announced that they have successfully received the manufacturing and sales approval for Adalimumab BS Subcutaneous Injection 20mg Syringe 0.2mL, 40mg Syringe 0.4mL, 80mg Syringe 0.8mL, 40mg Pen 0.4mL and 80mg Pen 0.8mL in the Japanese market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a biosimilar of the Adalimumab monoclonal antibody, Adalimumab BS has been instrumental in treating Rheumatoid Arthritis, Inflammatory Bowel Disease and other autoimmune conditions. It holds approval in 32 nations worldwide, encompassing South Korea, the US, and EU jurisdictions.
Both Nippon Kayaku and Celltrion are committed to encouraging the correct application of this medication, delivering pertinent data, and guaranteeing a consistent supply chain. Moreover, there's an emphasis on advocating for domestic biosimilar progression and striving to benefit patients, their relatives, and healthcare specialists.
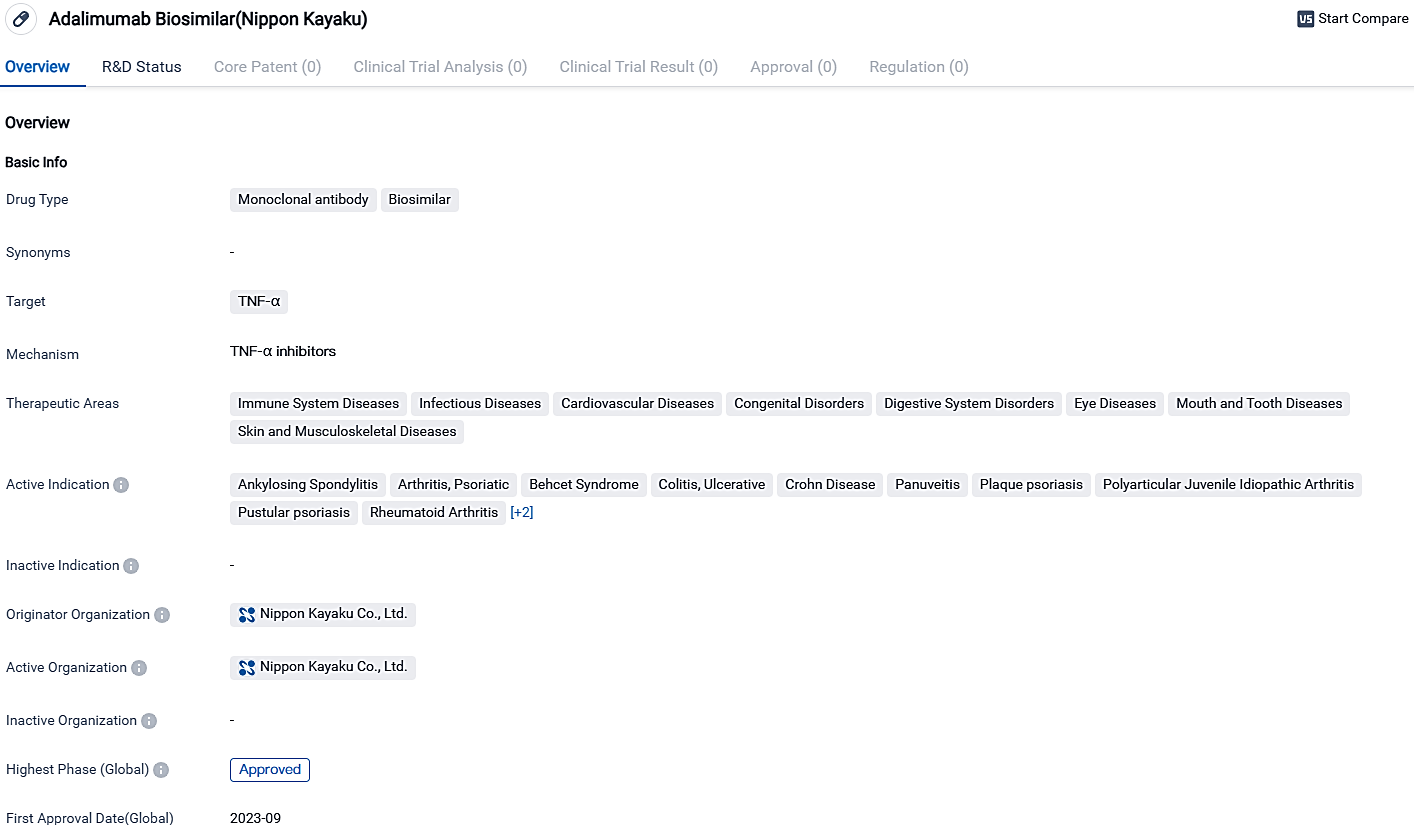
Adalimumab Biosimilar, which interacts with TNF-α, is certified for utilization across a wide spectrum of healthcare sectors, including conditions related to the immune and cardiovascular system, birth defects, gastrointestinal disorders, ophthalmological and dental issues, as well as skin and musculoskeletal ailments.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
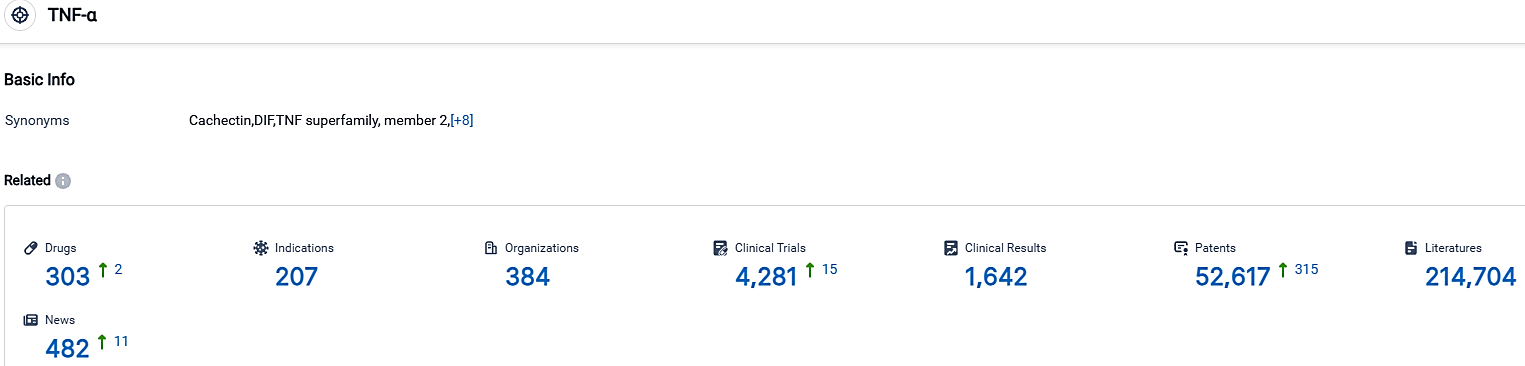
According to the data provided by the Synapse Database, As of October 1, 2023, there are 303 investigational drugs for the TNF-α, including 207 targets,384 R&D institutions involved, with related clinical trials reaching 4281,and as many as 52617 patents.
Adalimumab Biosimilar is employed to tackle diverse conditions including ankylosing spondylitis, psoriatic arthritis, ulcerative colitis, and rheumatoid arthritis. Its development is coming to fruition as it's reached its highest phase and is projected to receive approval by October 2023 within Japan.