Novartis research indicates that the oral drug remibrutinib can start easing chronic spontaneous urticaria symptoms by week two of treatment
Novartis has declared fresh encouraging results from its Phase III REMIX-1 and REMIX-2 experiments, exploring the efficacy of remibrutinib, a highly selective oral Bruton’s tyrosine kinase (BTK) inhibitor, on patients with chronic spontaneous urticaria who don't respond sufficiently to H1-antihistamines. The research revealed that remibrutinib achieved all the key and secondary objectives by Week 121.
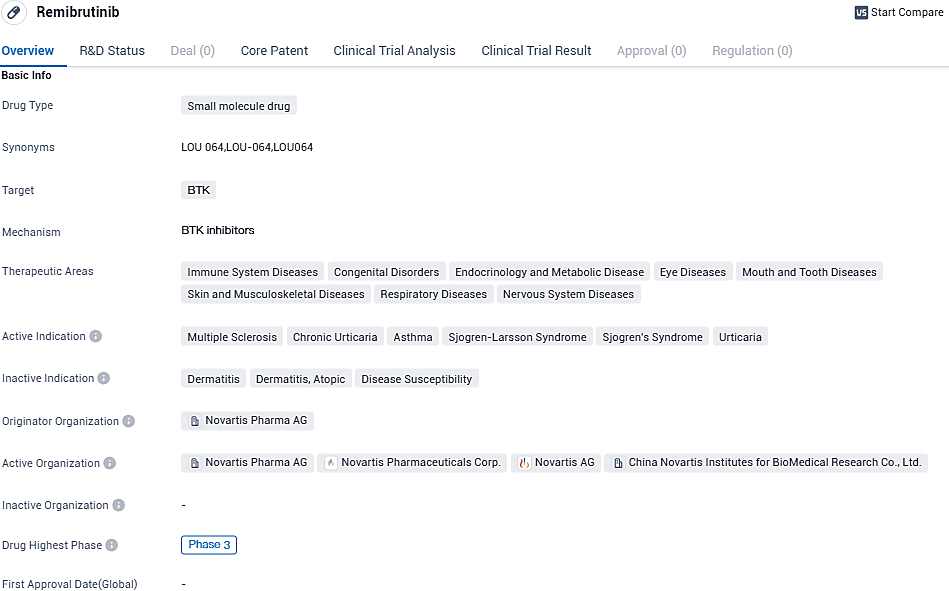
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The efficacy of Remibrutinib showed marked improvement from the baseline when compared with placebo for aspects like urticaria activity on a weekly basis, itch and hives up to week 121. It was observed that a significant proportion of patients displayed well-managed disease symptoms (UAS7≤6) through the use of Remibrutinib as compared to placebo.
This control was seen as early as the 2nd week and was sustained till the 12th week. Furthermore, nearly a third of the patients experienced complete relief from itch and hives at week 121. These findings will be made public at the 2023 American College of Allergy, Asthma and Immunology Scientific Meeting in Anaheim, California, scheduled for November 9–13.
Marcus Maurer, M.D., Professor of Dermatology and Allergy, and executive director at the Institute of Allergology at Charité – Universitätsmedizin Berlin, commented, “The significance of these observations should be highlighted especially as it bodes well for several individuals who are afflicted with CSU and continue to exhibit symptoms."
Maurer further added that living with CSU is quite demanding as it can affect various aspects of a patient’s life, including sleep patterns and work efficiency; hence, a potential alternative treatment option that might provide relief in as early as two weeks of mono-therapy with antihistamines could be a game-changer.
The Global Head of Development, Immunology at Novartis, Angelika Jahreis, too had something to say about the patients with CSU. He pointed out the limited treatment options available for CSU patients and admitted that the existing treatment, i.e., antihistamines, could not control symptoms even when dosed higher than the usual, leading to uncontrolled symptoms and side effects including drowsiness.
To conclude, Jahreis said, “I am thrilled about the prospect of offering a potential new treatment option for CSU patients who are consistently troubled by itching and an inhibited lifestyle. The data indicates that Remibrutinib, an oral BTKi, provided significant relief from symptoms in as early as the 2nd week and the improvement was constant till the 12th week.”
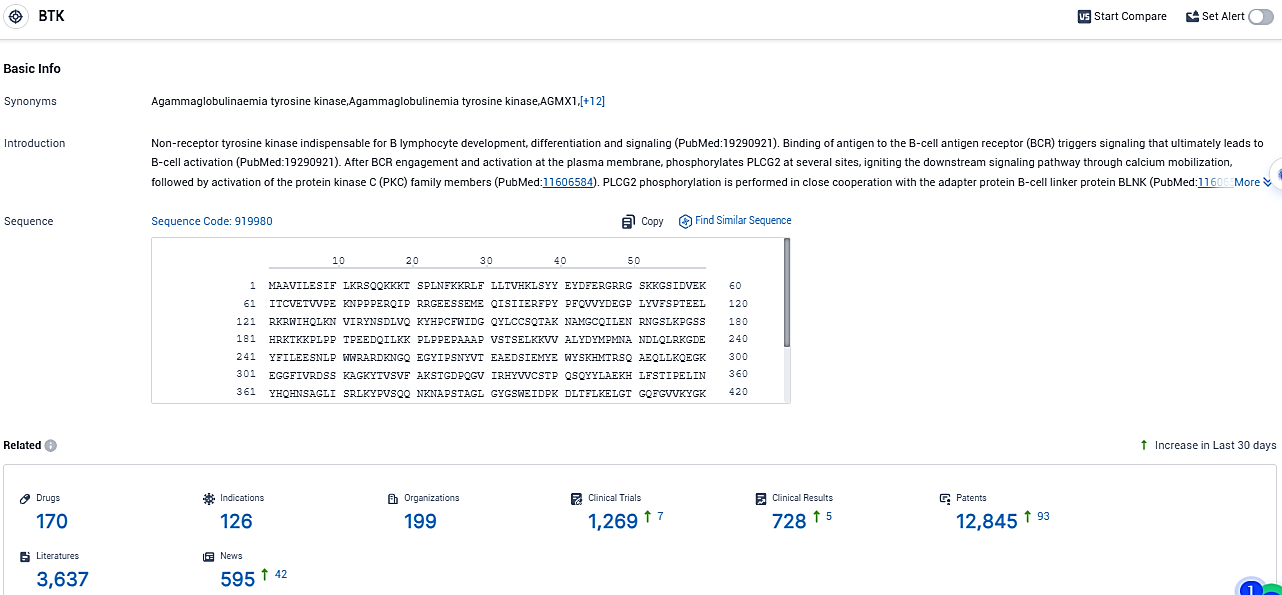
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 18, 2023, there are 170 investigational drugs for the BTK target, including 126 indications, 199 R&D institutions involved, with related clinical trials reaching 1269, and as many as 12845 patents.
Remibrutinib is an investigational highly selective, covalent, oral BTK inhibitor that blocks the BTK cascade and prevents the release of histamine that causes itchy hives and swelling. If approved, remibrutinib has the potential to become an effective oral option to complement Xolair® (omalizumab), the first and only injectable biologic indicated for CSU14. In the US, Novartis Pharmaceuticals Corporation and Genentech, a member of the Roche Group, work together to develop and co-promote Xolair.