Paclitaxel: brief review of its R&D progress and the clinical result in 2023 ESMO
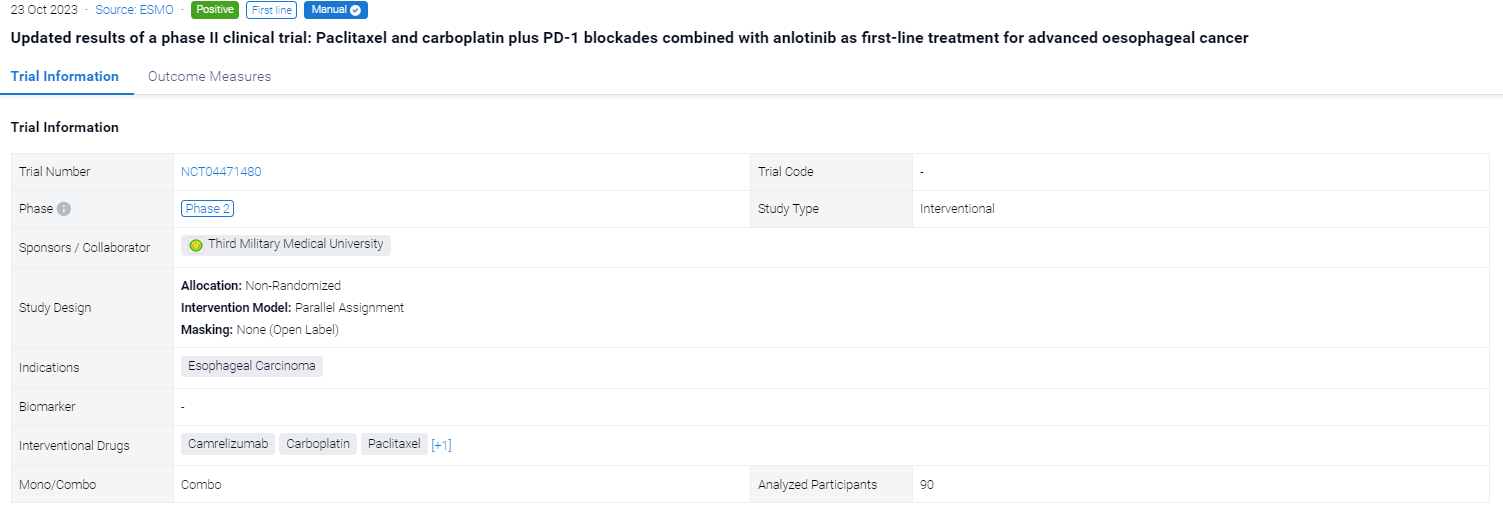
On 23 Oct 2023, the updated results of a phase II clinical trial: Paclitaxel and carboplatin plus PD-1 blockades combined with anlotinib as first-line treatment for advanced oesophageal cancer was reported at the ESMO Congress.
Paclitaxel's R&D Progress
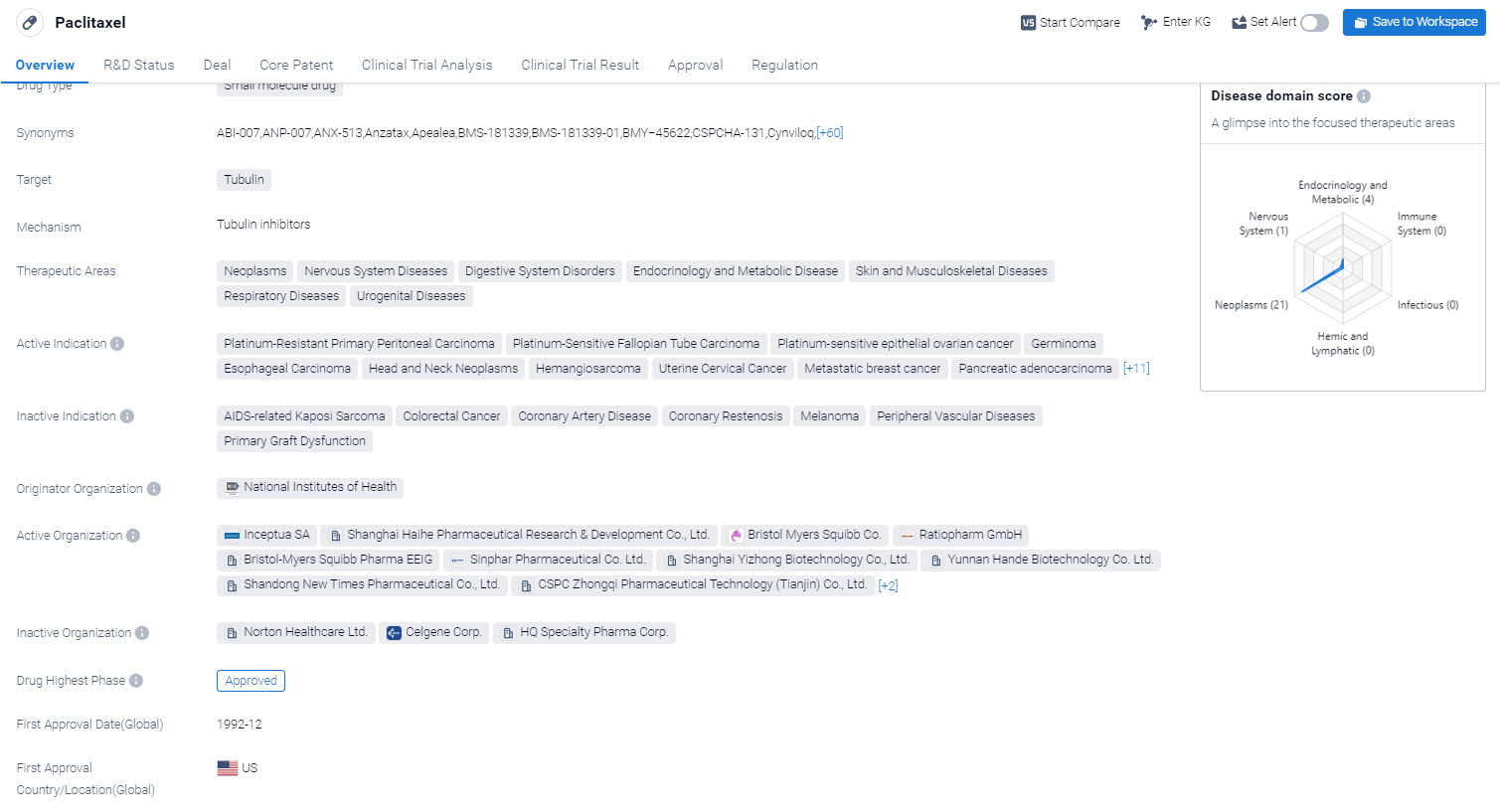
Paclitaxel is a small molecule drug that primarily targets tubulin, a protein involved in cell division. It has been approved for use in various therapeutic areas, including neoplasms (abnormal growth of cells), nervous system diseases, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, respiratory diseases, and urogenital diseases.
According to the Patsnap Synapse, paclitaxel has reached the highest phase of development, with global approvals. And the clinical trial areas for Paclitaxel are primarily in the United States, China, and United kingdom. The key indication is Metastatic breast cancer.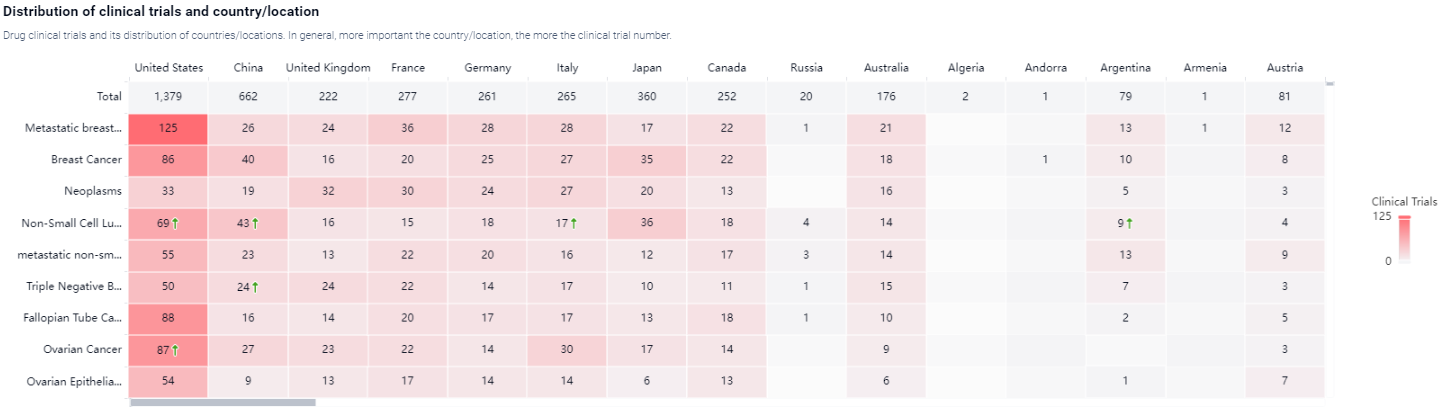
Detailed Clinical Result of Paclitaxel
The non-randomized, parallel assignment, open-labeled clinical trial (NCT04471480) was aimed to evacuate the efficacy of paclitaxel and carboplatin combined with anlotinib and PD-1 blockades in the treatment of advanced oesophageal cancer.
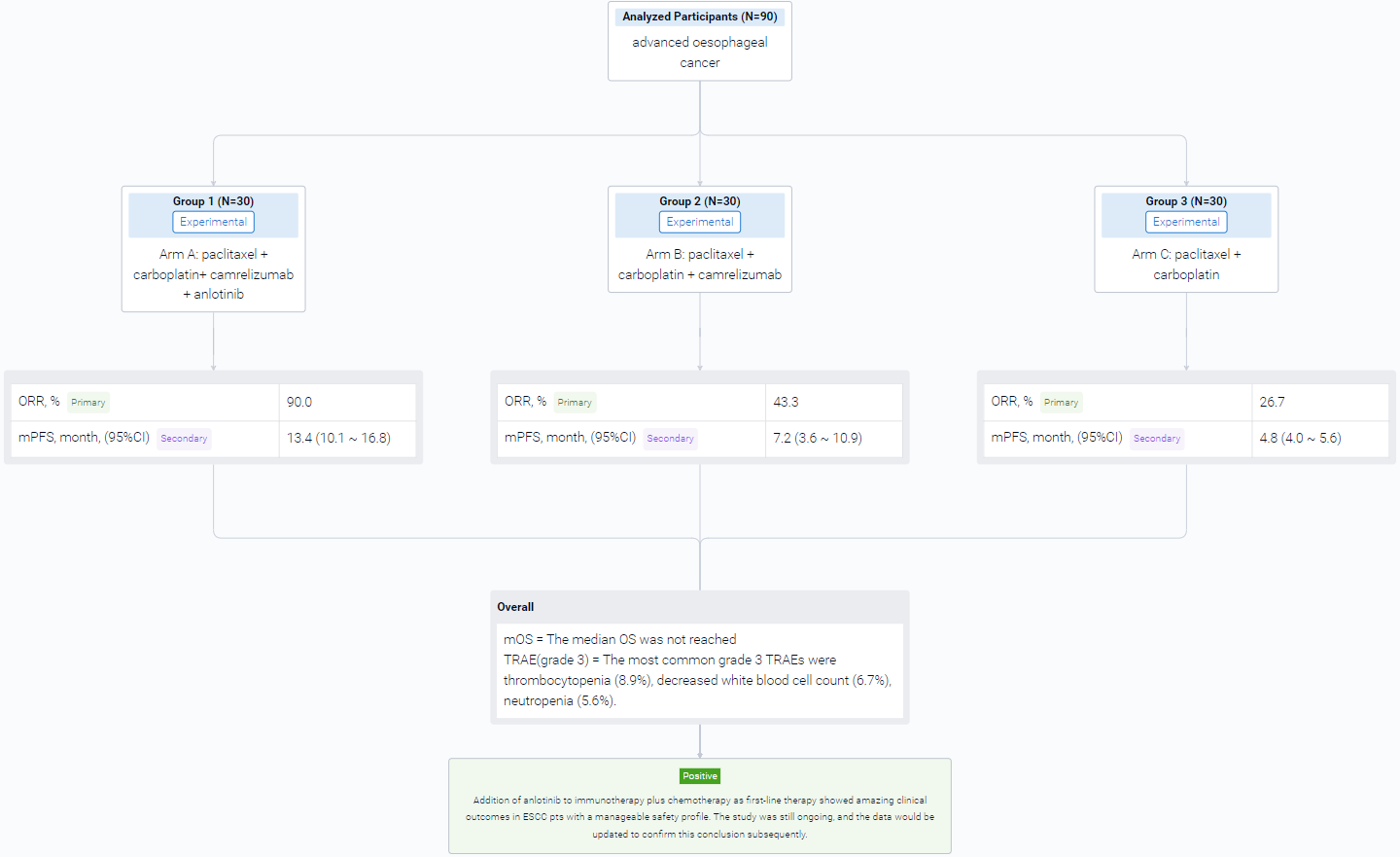
In this study, a total of 90 patients (pts) with previously untreated, advanced or metastatic ESCC, with an age ranging from 18-75 years old were planned to be enrolled into three arms with an allocation ratio of 1:1:1. Arm A received TC (paclitaxel + carboplatin) + camrelizumab (200mg, q3w) + anlotinib (8mg, q3w); Arm B received TC with camrelizumab; Arm C received TC only. After 4-6 cycles of induction therapy, arm A and arm B would receive maintenance therapy until disease progression or intolerable adverse events. The primary endpoint was ORR. Secondary endpoints included PFS, OS and safety.
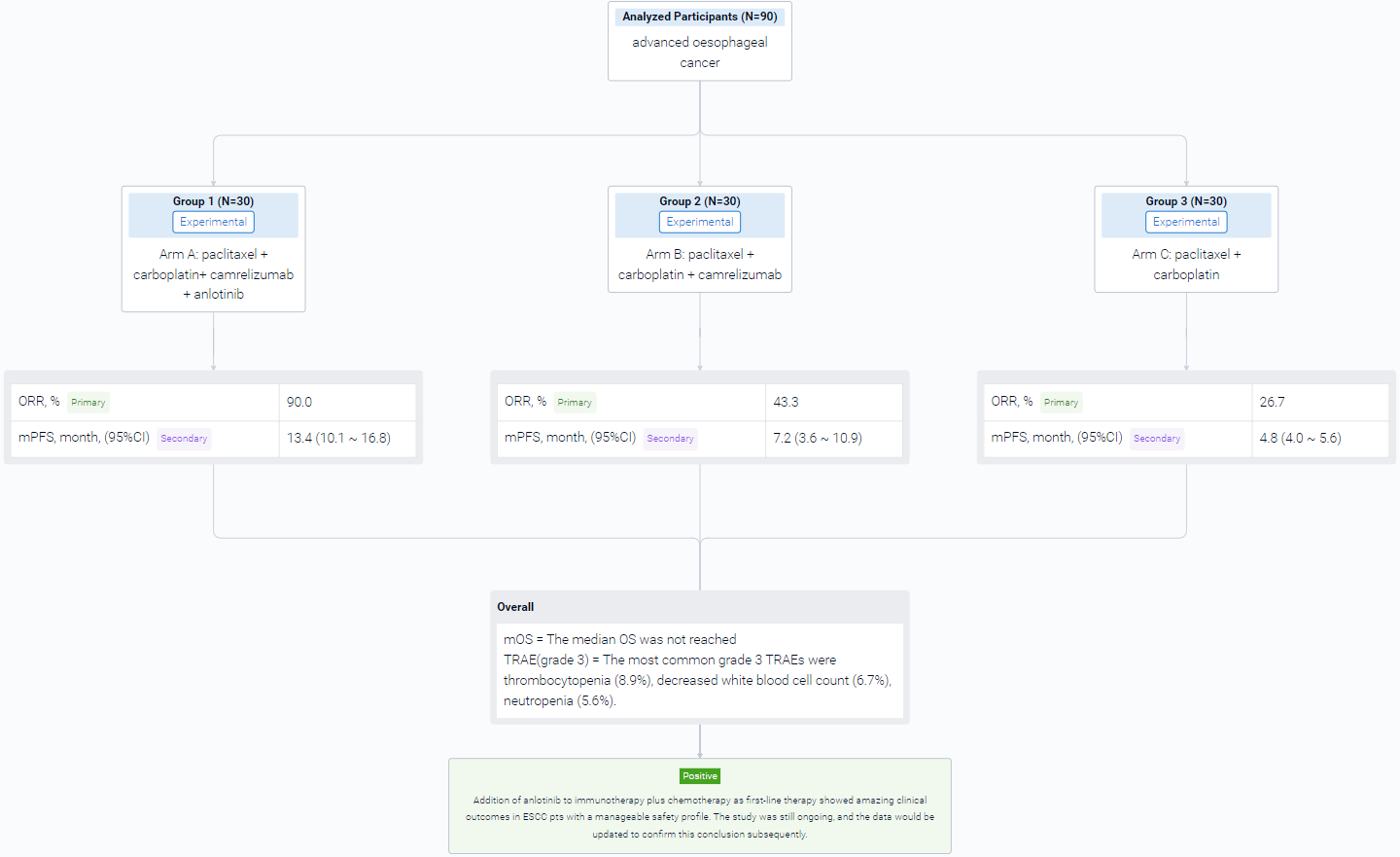
The result showed that at data cut-off date (Apr. 30, 2023), a total of 90 pts was enrolled. 25 of 30 pts achieved partial response (PR) and 2 pts achieved complete response in arm A, 13 of 30 pts achieved PR in arm B, and 7 of 30 pts achieved PR in arm C. The ORR were 90.0% in arm A, 43.3% in arm B, and 26.7% in arm C. The median PFS (95% CI) were 13.4 months (10.1-16.8) in arm A, 7.2 months (3.6-10.9) in arm B, and 4.8 months (4.0-5.6) in arm C. The median OS was not reached. The rate of treatment related adverse events (TRAEs) of any grade was 100% in all three groups, and grade 3 TRAEs were 30.0%, 23.3% and 13.3%, respectively. The most common grade 3 TRAEs were thrombocytopenia (8.9%), decreased white blood cell count (6.7%), neutropenia (5.6%). No grade 4 or 5 TRAEs were observed.
It can be concluded that Addition of anlotinib to immunotherapy plus chemotherapy as first-line therapy showed amazing clinical outcomes in ESCC pts with a manageable safety profile. The study was still ongoing, and the data would be updated to confirm this conclusion subsequently.
How to Easily View the Clinical Results Using Synapse Database?
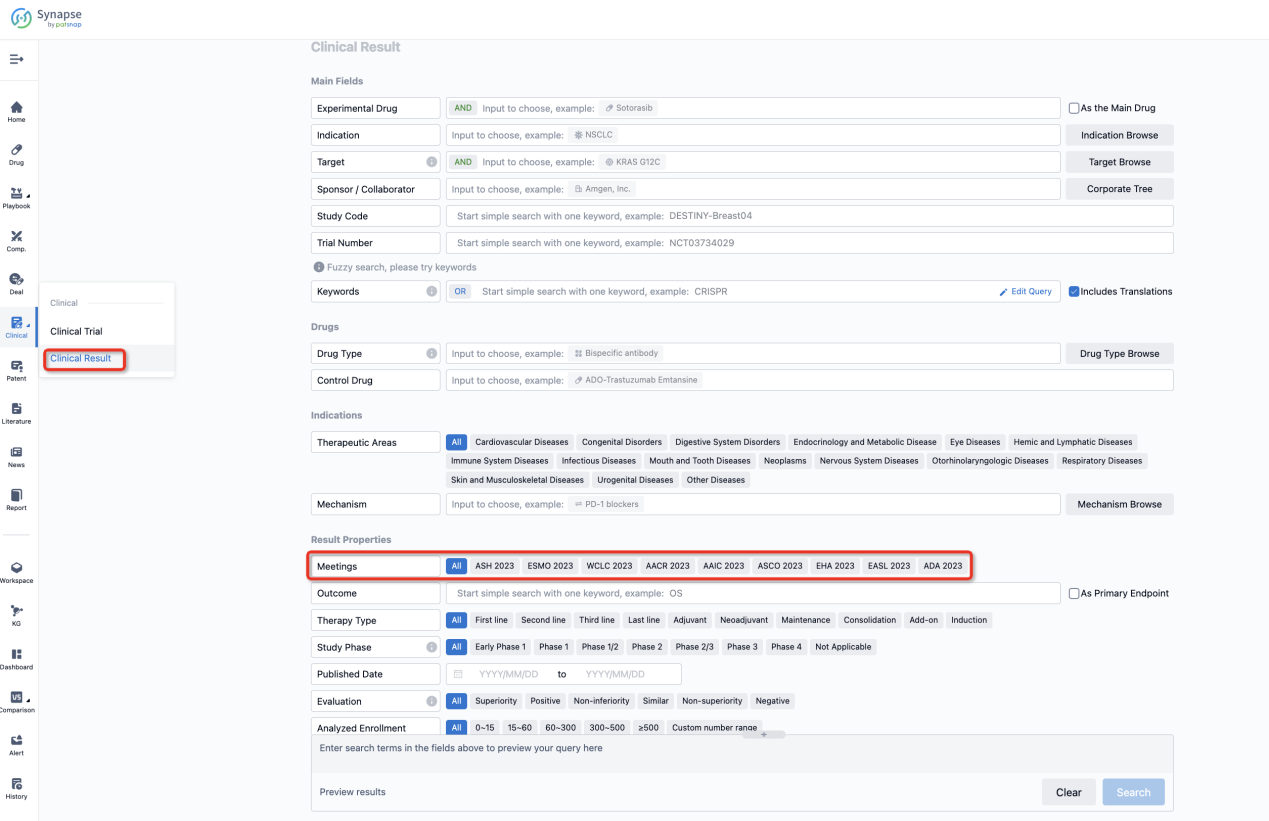
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
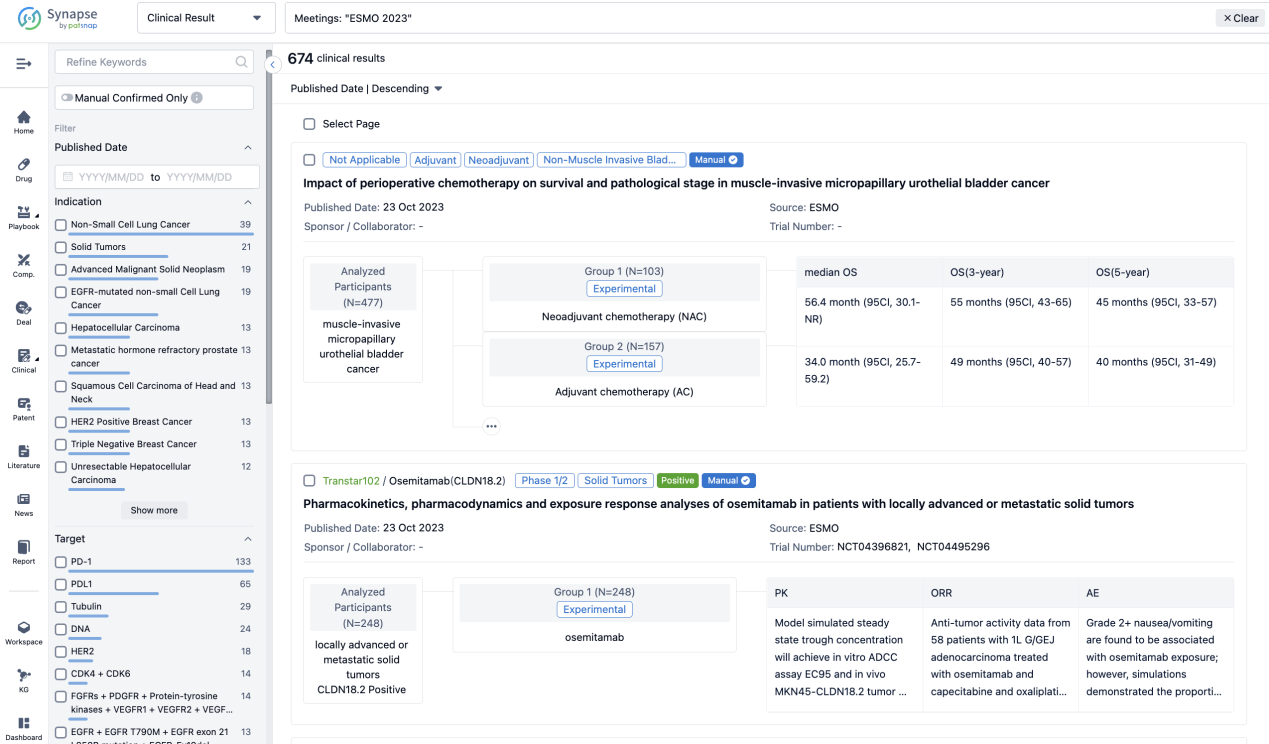
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
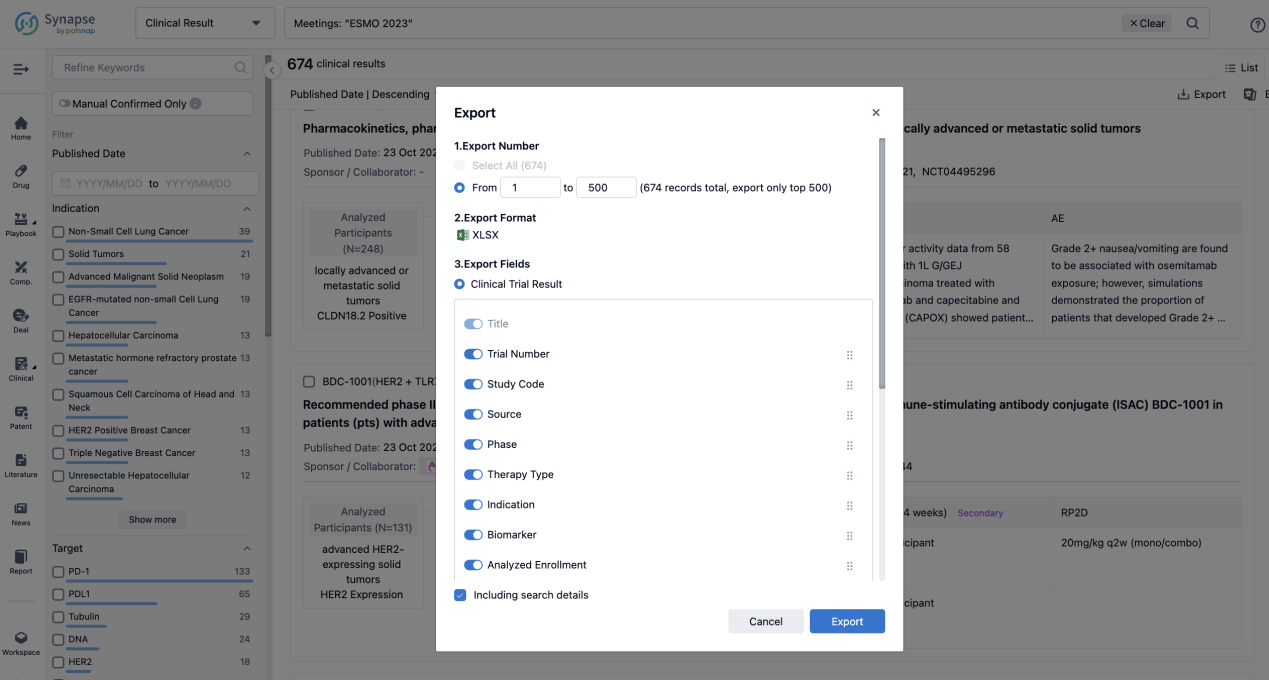
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!