Sana Biotech Reveals FDA Approval of New Drug Application for SC291
Sana Biotechnology, Inc., a firm dedicated to altering patient outcomes via engineered cells, has received an FDA clearance for its IND application, initiating a SC291 study for individuals suffering from numerous B-cell mediated autoimmune ailments. These include lupus nephritis, extrarenal lupus, and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 "Doug Williams, PhD, Sana’s President of R&D, stated, "We are in the process of creating SC291 for those suffering from several B-cell related autoimmune conditions, and receiving this IND approval signifies a vital achievement. SC291 is an allogeneic CAR T cell treatment, with an upscale production method capable of creating numerous patient doses in each run, which we think will be fundamental in tackling these considerable unaddressed necessities."
"Doug Williams, PhD, Sana’s President of R&D, stated, "We are in the process of creating SC291 for those suffering from several B-cell related autoimmune conditions, and receiving this IND approval signifies a vital achievement. SC291 is an allogeneic CAR T cell treatment, with an upscale production method capable of creating numerous patient doses in each run, which we think will be fundamental in tackling these considerable unaddressed necessities."
He continued, "Employing allogeneic cells further eases the treatment model for physicians and patients by doing away with apheresis and the need for individualized manufacturing of the medicinal product for each patient. We anticipate starting patient treatment soon and are expected to reveal preliminary safety and efficacy details covering various ailments in 2024."
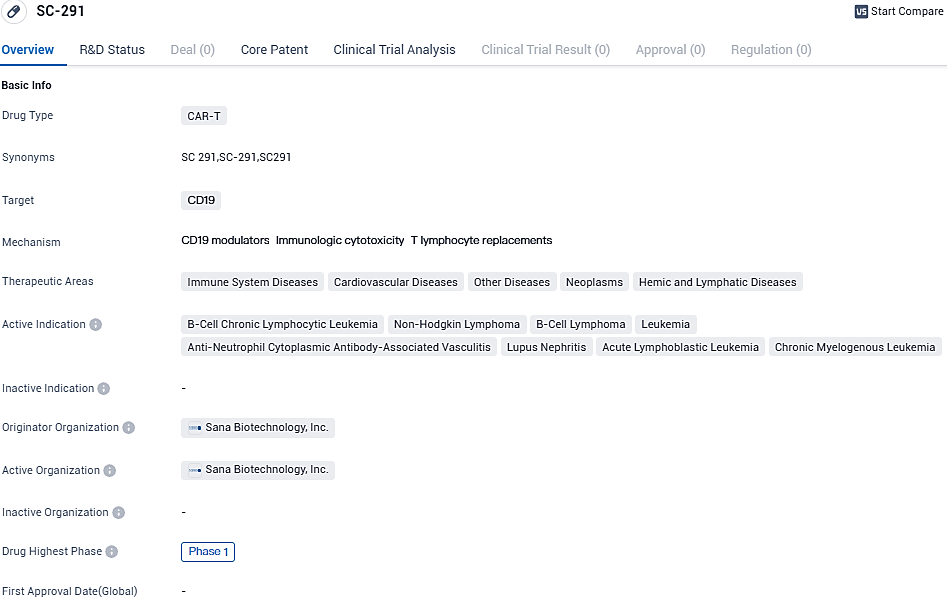
SC291, developed through Sana's hypoimmune platform, is a CD19-targeted allogeneic CAR T cell treatment. Our allogeneic T cell projects use T cells from healthy donors to produce CAR T treatments that, in this instance, aim at CD19, a protein found on the surface of B cells. B cells have been proven to lead to disease progression in many autoimmune conditions, and therapies targeting B cells from various modalities have been successful across several autoimmune conditions.
Fresh data in the domain affirms that an higher level of B cell eradication in tissues might correspond with better effectiveness and an acceptable safety profile. The introduction of CD19-directed CAR T treatment brings forth a new choice, where the CAR T serves as the effector cell eradicating B cells on-site. We plan on advancing SC291 in multiple scenarios, using our present hypoimmune allogeneic CAR T production platform, to amply cater to these significant unaddressed needs."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
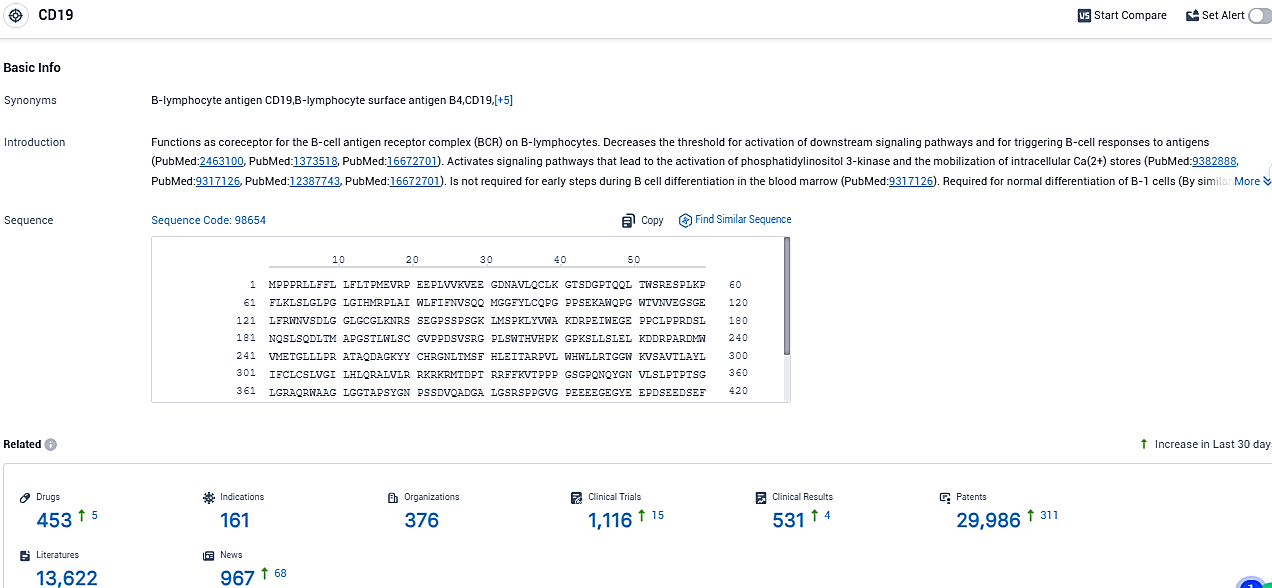
According to the data provided by the Synapse Database, As of November 18, 2023, there are 453 investigational drugs for the CD19 target, including 161 indications, 376 R&D institutions involved, with related clinical trials reaching 1116, and as many as 29986 patents.
SC-291 has the potential to be used in the treatment of various immune system diseases, cardiovascular diseases, neoplasms, and hemic and lymphatic diseases. The drug is currently in Phase 1 of clinical development, and further research is needed to determine its safety and efficacy.