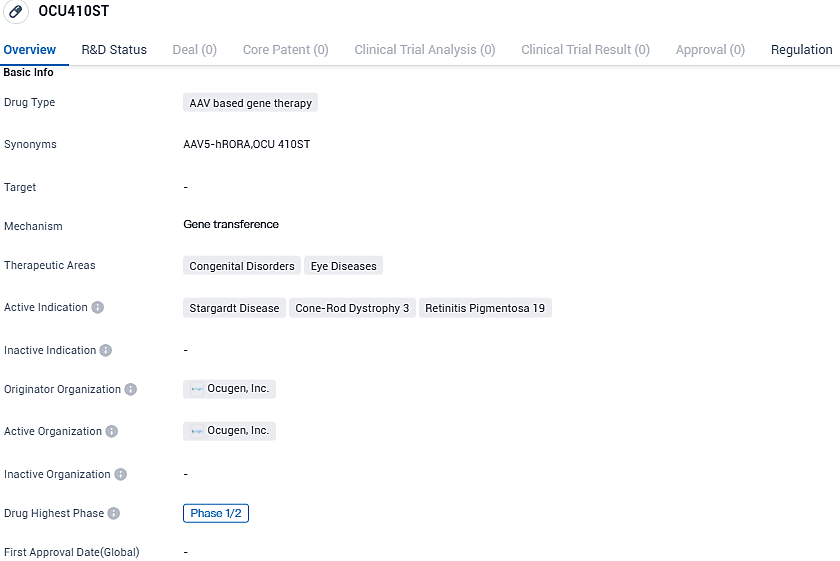
Ocugen declares commencement of Phase 1/2 trial after first administration on patient for OCU410ST
A biotech firm, Ocugen, Inc., specializing in the inception, advancement, and marketing of new gene and cell treatments, biologicals, and immunizations, revealed that they have initiated the first dosage in their stage one/two GARDian clinical examination of OCU410ST (AAV5-hRORA). This treatment is a potential modifier gene therapy for Stargardt disease, a scarce genetically passed disorder that primarily impacts the retina, frequently causing a gradual loss of vision in both children and adults.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"There's a significant gap in the medical landscape for close to 35,000 U.S. patients suffering from Stargardt disease," commented Dr. Shankar Musunuri, Chairman, CEO, and Co-Founder of Ocugen. "Our mission revolves around creating innovative healing methods for inherited retina-related conditions, and reaching this landmark is a momentous stride in delivering our groundbreaking modifier gene therapies to those with an urgent need."
This Phase 1/2 trial aims to evaluate the safety of unilateral subretinal inoculation of OCU410ST in Stargardt Disease sufferers and involves two key stages. Phase 1 involves a multi-center, open-label, dose-ranging investigation, while Phase 2 encompasses a randomized, outcome accessor-blinded, dose-expansion study where both adult and younger subjects will be assigned randomly at a ratio of 1:1:1 to one of two OCU410ST dosage sets or a non-treated control group.
OCU410ST employs an AAV delivery system to transport the RORA gene into the retina. It embodies Ocugen's modifier gene therapy approach, firmly rooted in the Nuclear Hormone Receptor RORA that governs path connections relevant to Stargardt disease like lipofuscin production, oxidative stress, compliment assignment, inflammation, and cellular survival systems.
"There's inherent value and intrigue in exploring groundbreaking therapies for incurable vision impairing conditions," noted Charles Wykoff, MD, PhD, Director of Research, Retina Consultants of Texas. "The launch of this trial procedure investigating a fresh mechanism of action for treating Stargardt disease is compelling and offers a ray of hope to patients and their families."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
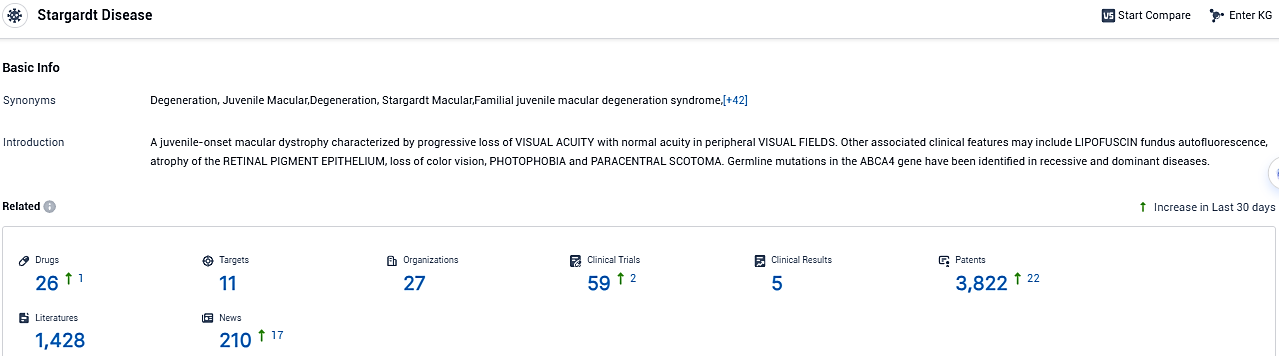
According to the data provided by the Synapse Database, As of November 18, 2023, there are 26 investigational drugs for the Stargardt disease, including 11 targets, 27 R&D institutions involved, with related clinical trials reaching 59, and as many as 3822 patents.
OCU410ST has been granted orphan drug status, which means it is intended to treat rare diseases that affect a small number of patients. This designation provides certain incentives and benefits to the drug developer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.