Roivant Reports Encouraging Preliminary Results for Phase 1 SAD and 300mg Under-skin IMVT-1402 MAD Trials
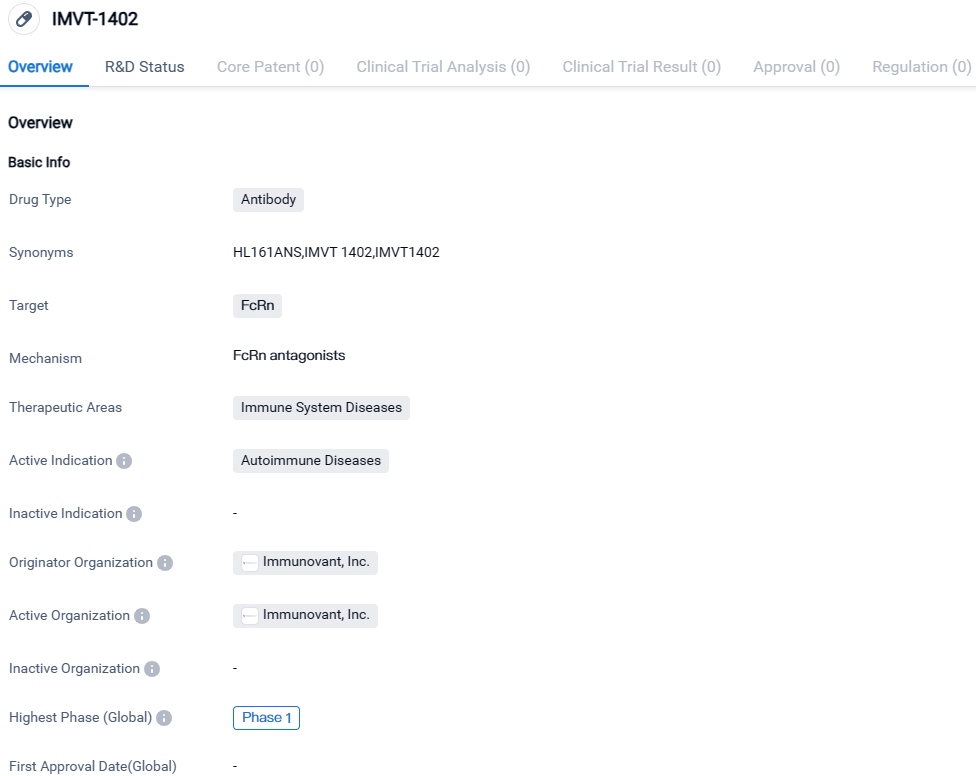
Immunovant, Inc., a corporation primarily engaged in the research of immunology and dedicated to improving the lives of those suffering from autoimmune disorders, recently revealed preliminary results of a Phase 1 clinical trial for IMVT-1402. The trial involves healthy adults and the data show that when it is administered subcutaneously, IMVT-1402 results in a dose-dependent decrease in IgG levels. There were no changes in concentrations of serum albumin or LDL-C that were associated with the dose, underscoring the potential of IMVT-1402 to be seen as a top-tier neonatal fragment crystallizable receptor (FcRn) inhibitor.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Pete Salzmann, M.D., CEO of Immunovant, has expressed optimism regarding the robust pharmacodynamic data acquired so far with IMVT-1402," "The initial human trials align well with previous non-human primate studies' findings, and we anticipate divulging more MAD data in November."
The initial phase of this clinical trial takes the form of a double-blind, randomized, ascending dose, placebo-controlled study and is designed to evaluate the safety, pharmacokinetics, tolerability, and pharmacodynamics of IMVT-1402 amongst healthy adults.
During the single-ascending dose section of the study, IMVT-1402, when applied subcutaneously, showcased a sustained IgG reduction equivalent to or exceeding that of batoclimab. Safety data were largely favorable, with all adverse effects being mild or moderate, and no significant serum albumin reduction from baseline or LDL-C increase observed at any timepoint measured.
Furthermore, Immunovant is delighted to reveal that the preliminary MAD study outcomes for the 300 mg group were unveiled earlier than planned today. The unveiled data encompass all the MAD data presently accessible. The 600 mg group dosage has recently been initiated. After weekly administrations of 300 mg SC doses of IMVT-1402, the MAD cohort witnessed a mean total IgG reduction from baseline of 63%, with no serum albumin or LDL-C fluctuation from baseline observed.
Subcutaneously administered IMVT-1402 to participants in this cohort as an uncomplicated 2 mL injection at a 150 mg/mL concentration resulted in mild to moderate treatment-emergent adverse events.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
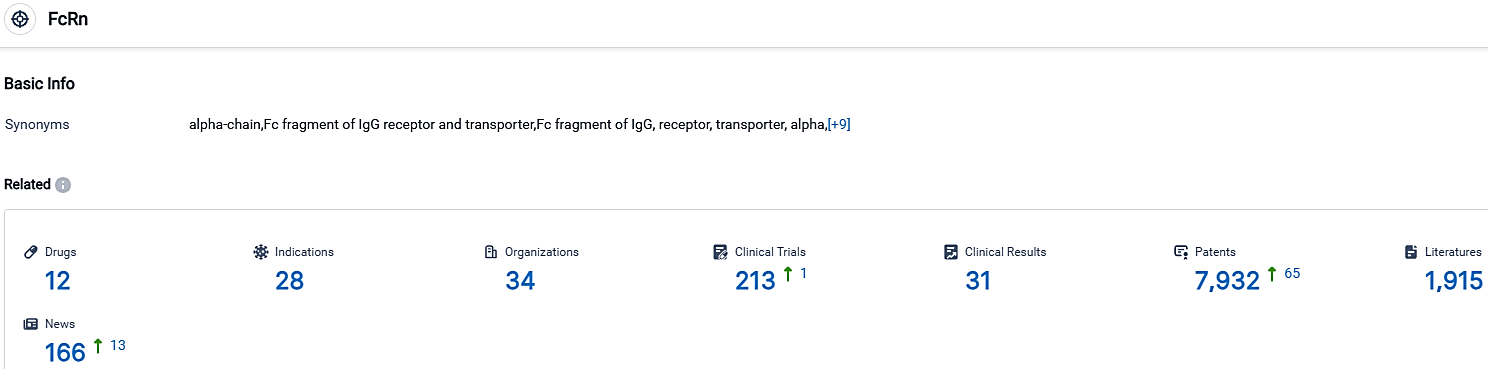
According to the data provided by the Synapse Database, As of October 1, 2023, there are 12 investigational drugs for the FcRn target, including 28 indications,34 R&D institutions involved, with related clinical trials reaching 213,and as many as 7932 patents.
IMVT-1402 is conceived as a potentially superior anti-FcRn antibody, targeted at the treatment of IgG-driven autoimmune disorders. Preliminary observations from a Phase 1 clinical trial with healthy subjects showed promising pharmacodynamic and safety metrics for IMVT-1402. Coupled with a user-friendly mode of application possibly allowing for self-administration by the patient, IMVT-1402 is well-positioned to be a potential remedy for a range of autoimmune diseases that are current areas of unmet patient need.