Palleon Pharma Presents GLIMMER-01 Findings on E-602 and Cemiplimab for Solid Tumors at SITC Meeting
Palleon Pharmaceuticals, a company in the clinical development phase that is at the forefront of glyco-immunology therapeutics for autoimmune disorders and cancer treatment, has announced the findings from the Phase 1/2 GLIMMER-01 study. This study evaluates E-602, their novel human sialidase enzyme therapy, administered alongside the PD-1 inhibitor cemiplimab (Libtayo®) in patients exhibiting PD-(L)1 resistance in solid tumors. These findings will be presented in both rapid oral and poster formats on Saturday, November 9, at the 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting taking place in Houston, Texas.
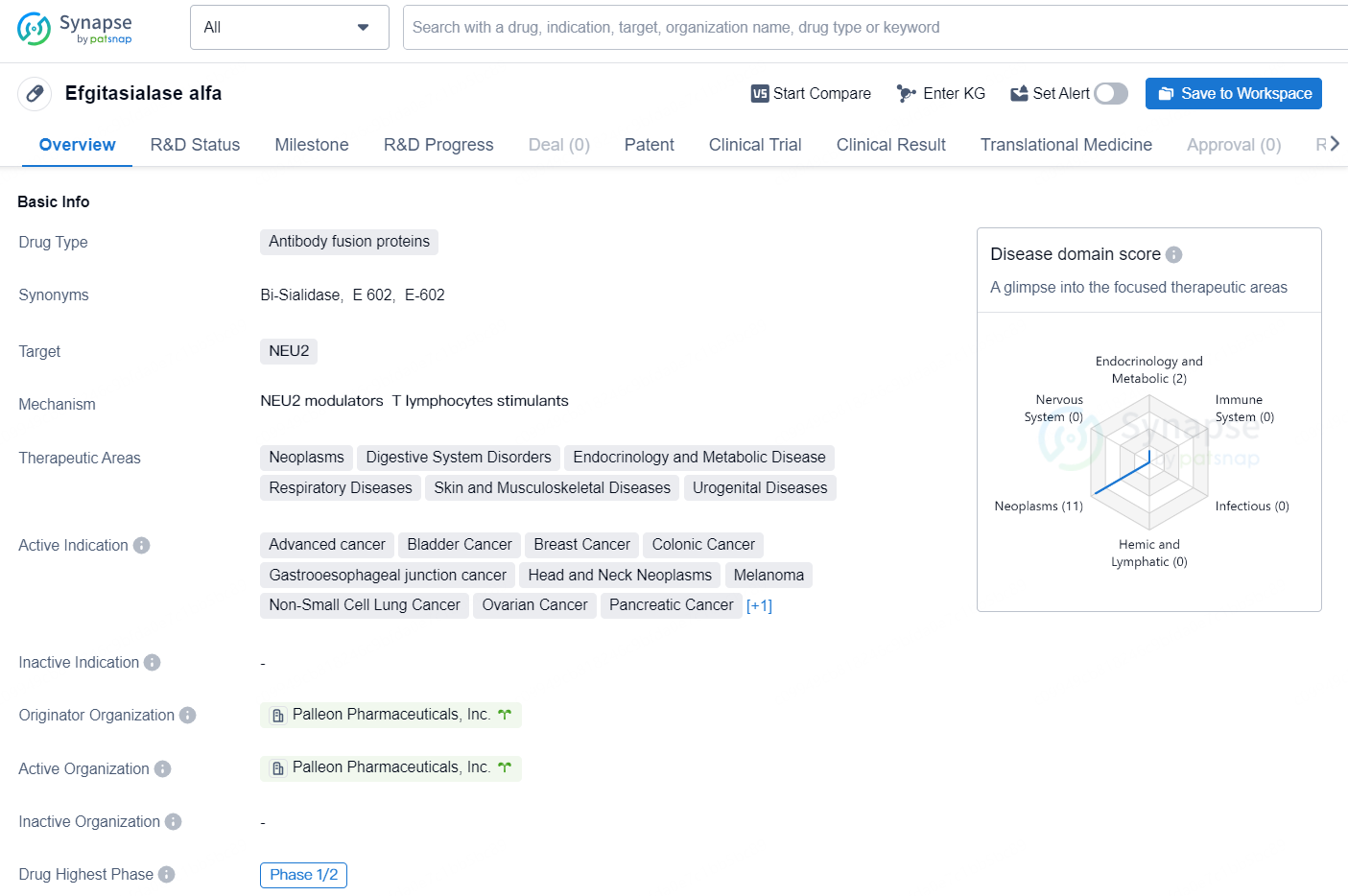
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In the Phase 1/2 clinical trial, a total of 21 patients diagnosed with anti-PD(L)-1-resistant melanoma, non-small cell lung cancer, and esophagogastric junction cancer, classified under the SITC consensus definition for immunotherapy resistance, were administered E-602 in conjunction with cemiplimab. Each patient underwent evaluation for tumor sialoglycan levels, determining whether they exhibited hypersialylation. The treatment combination was generally well-tolerated, with no observed dose-limiting toxicities. Patients demonstrating tumor hypersialylation showed a trend towards improved clinical outcomes compared to those without hypersialylation; notably, one patient with anti-PD-1 resistant melanoma achieved a confirmed partial response, and six others experienced disease stabilization. In contrast, all patients who did not exhibit hypersialylation experienced disease progression. Furthermore, paired tumor biopsies from those with hypersialylation indicated both tumor desialylation and immune modulation; however, the effects of tumor desialylation appeared to be temporary, lasting only 2-3 days with weekly administration. These results offer initial evidence supporting glycan editing as an innovative therapeutic strategy for immune system modulation.
“The evidence of mechanism and antitumor activity seen with E-602 as a combination treatment for patients with solid tumors strengthens the potential of targeting glyco-immunology to enhance the immune response in the treatment of cancer and autoimmune disorders,” stated Li Peng, Ph.D., Chief Scientific Officer of Palleon Pharmaceuticals. “We are eager to use E-602 for conditions that match its pharmacological properties and to advance our next-generation EAGLE molecules, which feature an extended half-life sialidase and a tumor-targeting component to meet the needs of cancer patients.”
“E-602 represents the inaugural candidate in a novel class of therapeutics aimed at modulating the immune response by focusing on glyco-immunology. Palleon is developing a robust pipeline of first-in-class drug candidates that hold the promise to enhance the quality of life and longevity of patients suffering from immune dysfunction diseases, such as cancer and autoimmunity,” remarked Jim Broderick, M.D., CEO and Founder of Palleon.
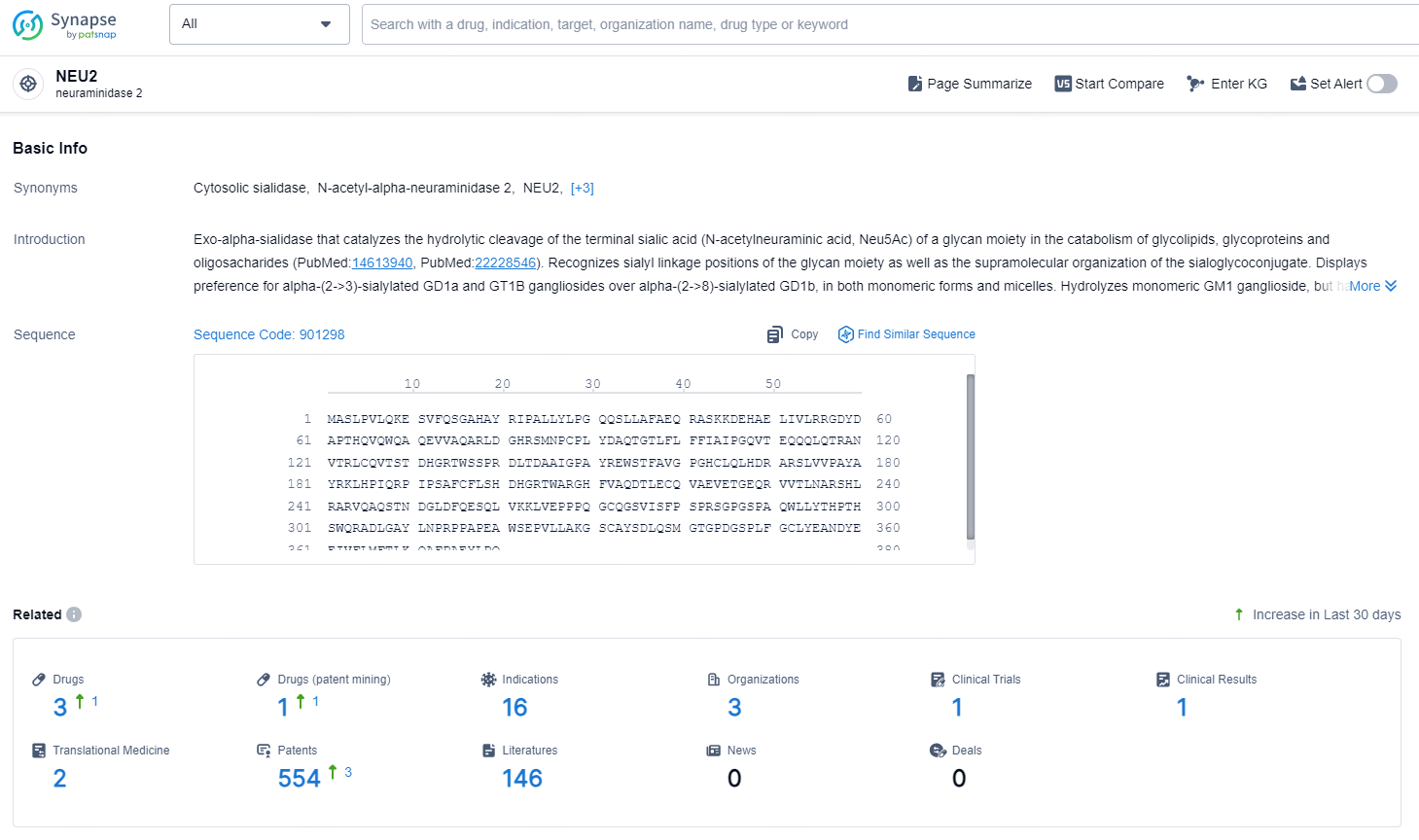
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 11, 2024, there are 3 investigational drugs for the NEU2 target, including 16 indication, 3 R&D institutions involved, with related clinical trial reaching 1, and as many as 554 patents.
Efgitasialase alfa is a type of antibody fusion protein drug that targets NEU2. It is being developed for the treatment of various therapeutic areas, including Neoplasms, Digestive System Disorders, Endocrinology and Metabolic Disease, Respiratory Diseases, Skin and Musculoskeletal Diseases, and Urogenital Diseases.