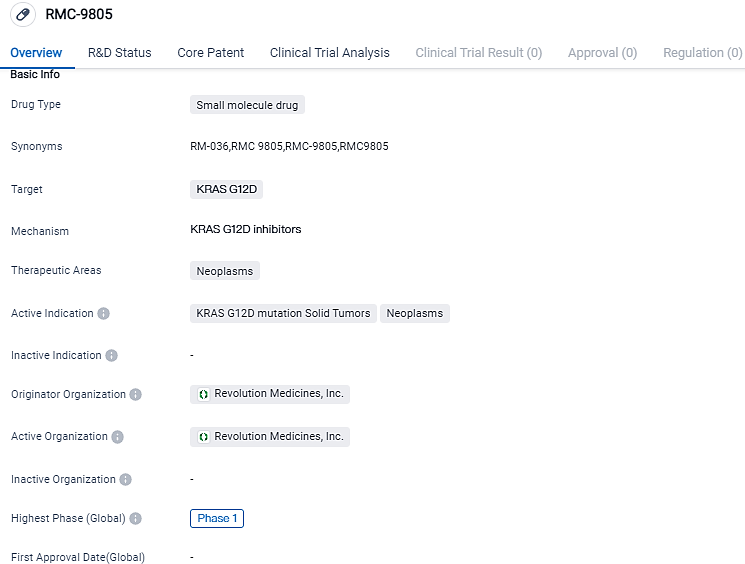
Revolution Medicines initiates dosing in Phase 1/1b clinical study for RMC-9805, an orally administered, covalent, mutant-specific KRASG12D inhibitor
Revolution Medicines, Inc., a company working in the oncology field and conducting clinical trials for RAS-dependent cancer treatments, has today confirmed that the initial patient has received a dose in their Phase 1/1b solo-therapy clinical research of RMC-9805. This KRAS G12D inhibitor is a covalent, orally-administered, selective mutation treatment engineered for assisting patients whose cancers are driven by the KRAS G12D mutation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
KRAS G12D has been identified as the leading driver in RAS-addicted human cancers, responsible for nearly 55,000 new diagnoses in the U.S. annually. It is predominantly found in patients with pancreatic cancer, NSCLC, and colorectal cancer.
In a multicenter, open-label Phase 1/1b trial, a dose-escalation and dose-expansion study will be carried out with RMC-9805. The trial's primary goals are to assess the compound's safety and tolerability, and establish the recommended Phase 2 dosage and schedule for RMC-9805 on patients with advanced solid tumors that harbor the KRAS G12D mutation.
Mark A. Goldsmith, M.D., Ph.D., CEO and Chairman of Revolution Medicines, announced that beginning patient dosing with RMC-9805 represents an important breakthrough for the company as it is their third oral RAS inhibitor to undergo clinical evaluation.
Dr. Goldsmith further elaborated, stating the robust clinical portfolio and an extensive collection of potential mutant-selective drug candidates and research-stage assets give them hope that they can transform conventional treatment approaches for patients struggling with different types of RAS-addicted cancers, such as NSCLC, pancreatic and colorectal cancers.
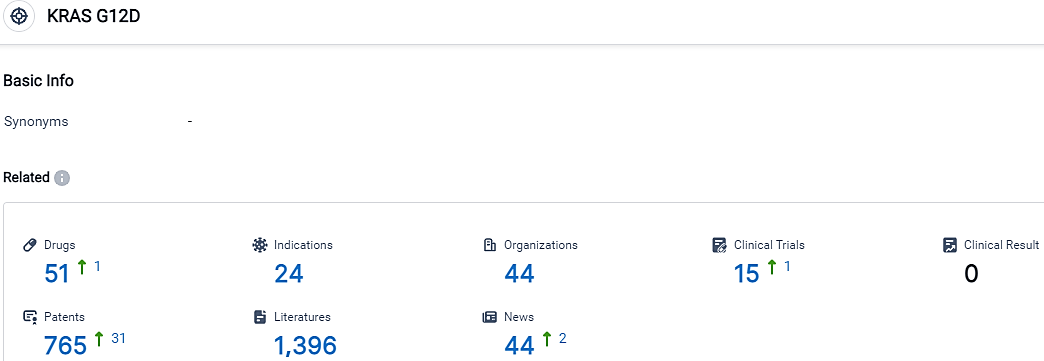
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 25, 2023, there are 51 investigational drugs for the KRAS G12D target, including 24 indications,44 R&D institutions involved, with related clinical trials reaching 15,and as many as 765 patents.
Further research and clinical trials are necessary to determine the safety, efficacy, and potential side effects of RMC-9805. As the drug is currently in Phase 1, it is still in the early stages of development, and its ultimate success and approval for commercial use will depend on the outcomes of further clinical trials.