Sapience Therapeutics announces ST101 clinical results at the 2023 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics
Sapience Therapeutics, Inc., a biotech firm at the clinical stage, concentrating on the exploration and production of peptide treatments which tackle oncogenic and immune imbalance contributing to cancer, has stated that an abstract about ST101 has been given the green light to be presented as a poster at the future AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, happening from October 11-15, 2023 in Boston, MA.
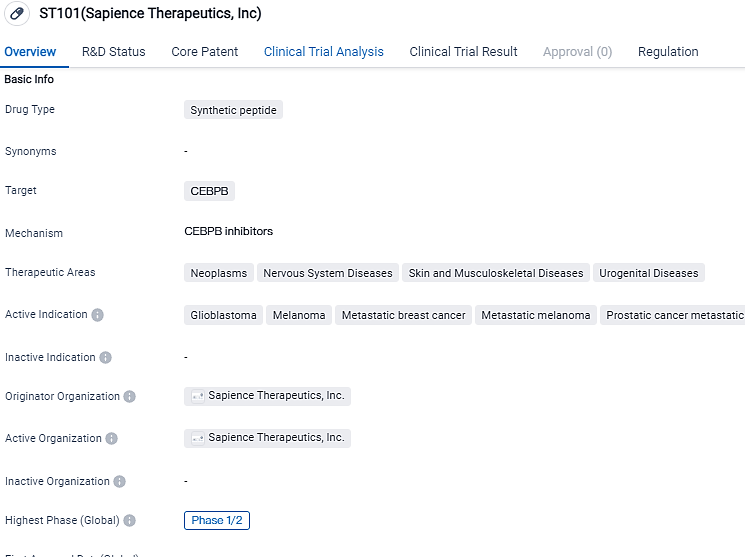
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ST101, a unique C/EBPβ antagonist, has presented clinical evidence of efficacy in advanced solid tumors. Currently, ST101 is under investigation in the Phase 2 segment of an ongoing Phase 1-2 clinical research involving patients with advance, inoperable, and metastatic solid tumors. The forthcoming poster presentation will compile clinical information from the reoccurring Glioblastoma Phase 2 cohort of 30 patients involving ST101.
Sapience Therapeutics Inc. is a privately operated, clinical-stage bio-tech firm that is focused on the creation and discovery of peptide-based therapeutics for cancer-related oncogenic and immune dysregulation. The firm's proprietary discovery platform optimizes the rational design of innovative peptides suitable for clinical development.
By utilizing our discovery potential, Sapience has created a range of therapeutic candidates known as SPEARs™, which obstruct intracellular protein-protein interactions, allowing the targeting of transcription factors traditionally deemed as undruggable. Sapience is pushing forward its primary programs, ST316, a front-runner in β-catenin antagonists, and ST101, a first-time C/EBPβ antagonist, via Phase 1-2 clinical trials.
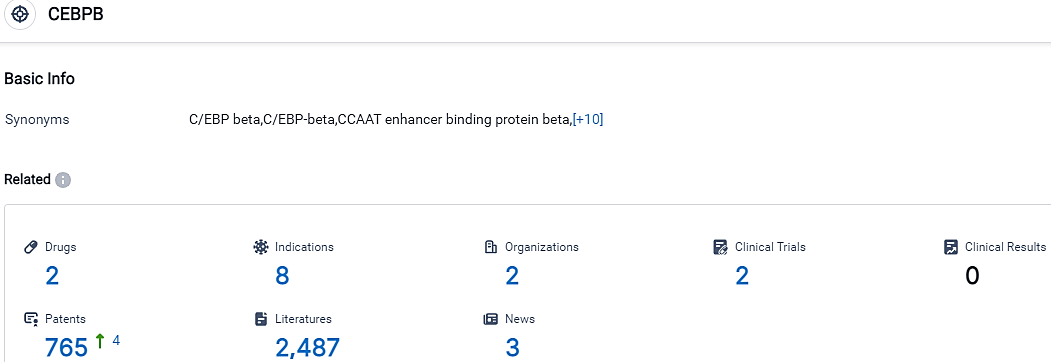
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 2 investigational drugs for the C/EBPβ target, including 8 indications,2 R&D institutions involved, with related clinical trials reaching 2,and as many as 765 patents.
ST101 presents potential as a prospective remedy for diverse cancer forms. The drug's synthetic peptide constituency and its focus on CEBPB reflects an innovative strategy within the realm of biomedicine. The therapeutic activation and regulatory credentials awarded to the drug accentuate its prospective role in responding to unresolved healthcare demands.