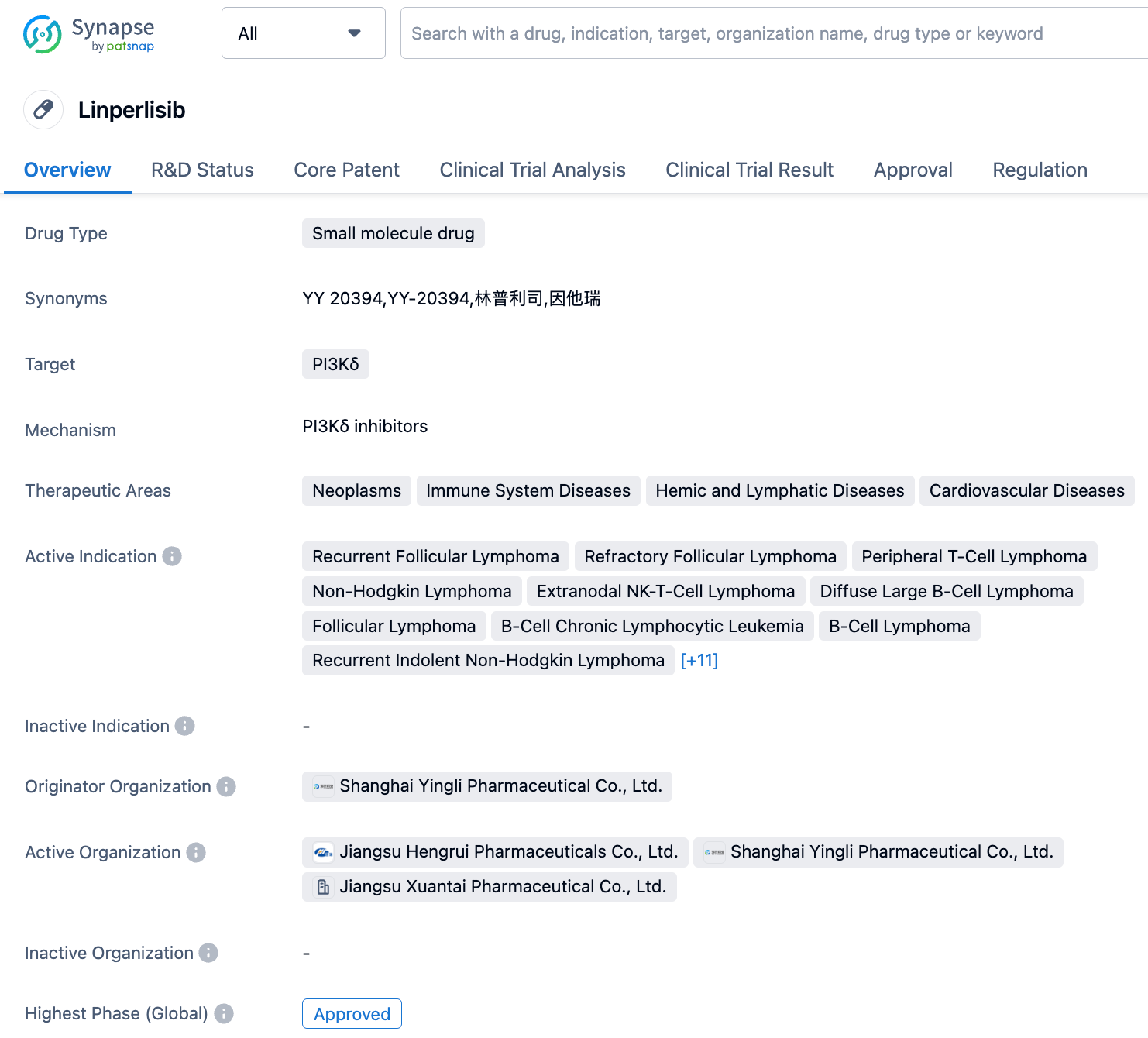
Shanghai Yingli Pharmaceutical submits a marketing application for PI3Kδ inhibitor, Linperlisib, for new indication for the treatment of Peripheral T-Cell Lymphoma
On September 6, 2023, Shanghai Yingli Pharmaceutical announced that its PI3Kδ inhibitor, Linperlisib, has been accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration of China for a new indication filing. This is for the treatment of patients with relapsed and/or refractory peripheral T-cell lymphoma (R/R PTCL). This is the second indication for which Linperlisib has been filed for marketing approval.
Linperlisib is a class one innovative drug independently developed by Shanghai Yingli and owns the intellectual property rights. It is a new generation of small molecule inhibitors of phosphoinositide 3-kinase delta (PI3Kδ). In February 2021, Jiangsu Hengrui Pharmaceuticals entered into a strategic cooperation agreement with Shanghai Yingli. Jiangsu Hengrui will invest 20 million US dollars in equity in Shanghai Yingli, and Shanghai Yingli grants Jiangsu Hengrui co-development rights and exclusive commercialization rights for Linperlisib in the Greater China region. On November 9, 2022, Linperlisib tablets were conditionally approved for marketing by the National Medical Products Administration of China for adult patients with relapsed or refractory follicular lymphoma who have previously received at least two systemic treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Previously, the results of a multicentric, single-arm, open-label Phase 1b study of Linperlisib in treating 43 instances of R/R PTCL (relapsed/refractory peripheral T-cell lymphoma) were announced at the 2022 annual meeting of the American Society of Hematology (ASH). The results showed that at a median follow-up of 17 months, the overall response rate (ORR) was 60%, the complete response (CR) rate was 35%, and the disease control rate (DCR) was 84%, indicating good safety.
The new indication submission for Linperlisib is based on the results of a single-arm, multicenter Phase 2 registration clinical study. This study aimed to evaluate the efficacy and safety of Linperlisib in treating R/R PTCL. The main endpoint of this study was the overall response rate, as evaluated by an Independent Review Committee (IRC).
Preliminary data showed that Linperlisib is effective and has manageable safety for treating R/R PTCL, and thus is likely to become a new treatment option for patients with R/R PTCL.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
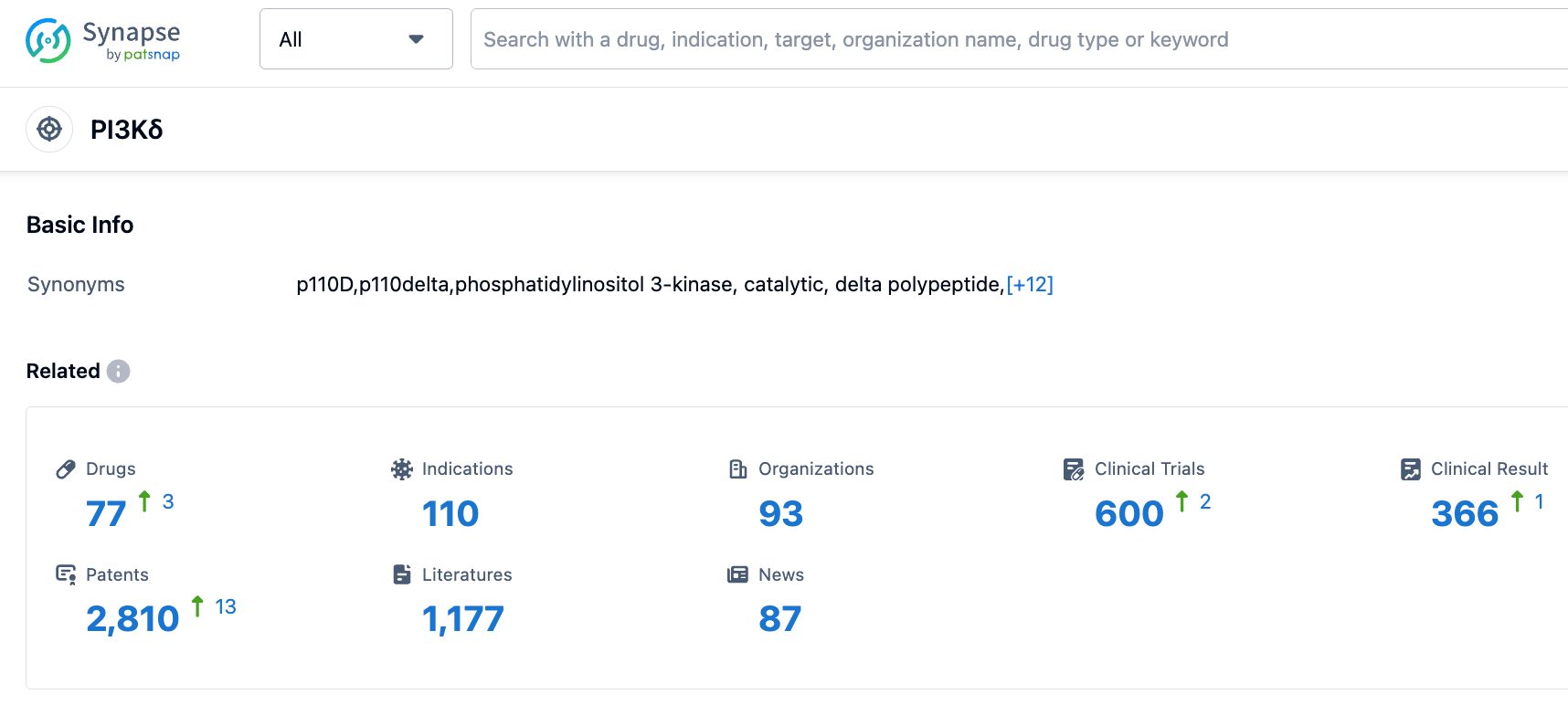
According to information disclosed by the Synapse database, as of September 8, 2023, there are a total of 77 investigational drugs for the PI3Kδ target, covering 110 indications, researched by 93 institutions, involving 600 related clinical trials, and up to 2811 patents.
Regarding the competitive landscape of PI3Kδ small molecule inhibitors in China, apart from Linperlisib, Duvelisib, introduced by CSPC Pharmaceutical from Verastem, was approved for marketing in March 2022. The indication is for the treatment of relapsed or refractory (R/R) follicular lymphoma (FL) that has undergone at least two previous systemic treatments. Bayer's Copanlisib Dihydrochloride was also approved for marketing in May 2023 with the same indication for R/R FL. The competition in this field will become more intense in the future.