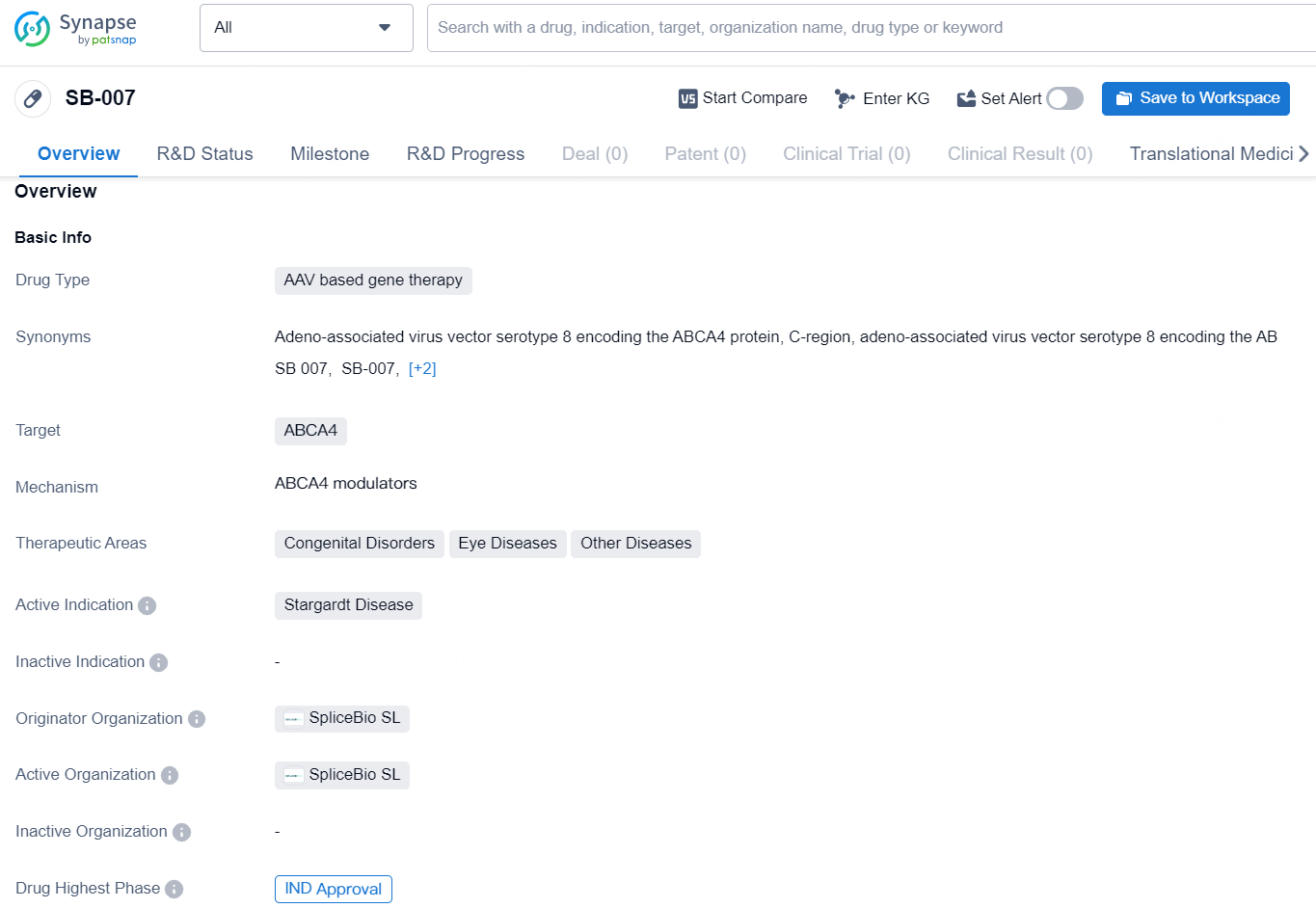
SpliceBio Gets FDA Clearance for SB-007 in Stargardt Disease Trials
SpliceBio, a company focused on genetic therapies and leading the way in Protein Splicing to tackle disorders resulting from mutations in substantial genes, has revealed that the U.S. Food and Drug Administration (FDA) has approved its investigational new drug (IND) application for its primary program, SB-007. This therapeutic, SB-007, is currently the sole clinical-stage treatment targeting the fundamental genetic cause of Stargardt disease, offering potential treatment options for all patients with various ABCA4 mutations.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Miquel Vila-Perelló, Ph.D., the Chief Executive Officer and Co-Founder of SpliceBio, remarked, “The FDA's IND approval of SB-007 marks a pivotal moment for both SpliceBio and individuals affected by Stargardt disease. Being the first IND granted for a Protein Splicing gene therapy, this breakthrough showcases the potential of this innovative therapeutic modality to tackle diseases linked to mutations in large genes like ABCA4. SB-007 is a gene therapy using an adeno-associated viral (AAV) vector designed to restore the expression of the full-length ABCA4 protein; it is the only clinical-stage therapy that has the ability to benefit all patients suffering from Stargardt disease. We are eager to expedite the clinical development of SB-007, leveraging the Orphan Drug Designation awarded by the FDA in 2024, and advancing this potentially life-altering therapy for Stargardt disease patients.”
“Developing gene therapies for Stargardt disease has posed challenges primarily due to the large size of the ABCA4 gene, and there are currently no approved treatments available,” stated Professor Paul Yang, M.D., Ph.D., head of the Paul H. Casey Ophthalmic Genetics Division at the Casey Eye Institute of Oregon Health & Science University. “This novel therapy adopts a distinct method to replace the complete, normal ABCA4 protein with high efficiency, effectively targeting the underlying cause of Stargardt disease across all pathogenic variants in the ABCA4 gene. The clearance of this IND signifies a significant advancement in the field, and I am excited to participate in the clinical trials investigating this promising strategy that could change the lives of those with Stargardt disease.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 20, 2024, there are 18 investigational drugs for the ABCA4 target, including 10 indications, 20 R&D institutions involved, with related clinical trials reaching 7, and as many as 1278 patents.
SB-007 is an AAV-based gene therapy drug developed by SpliceBio SL, targeting the ABCA4 gene. The drug falls within the therapeutic areas of congenital disorders, eye diseases, and other diseases, with a specific focus on treating Stargardt Disease. As of current information available, SB-007 has received IND (Investigational New Drug) approval, signifying its progression to clinical trials.