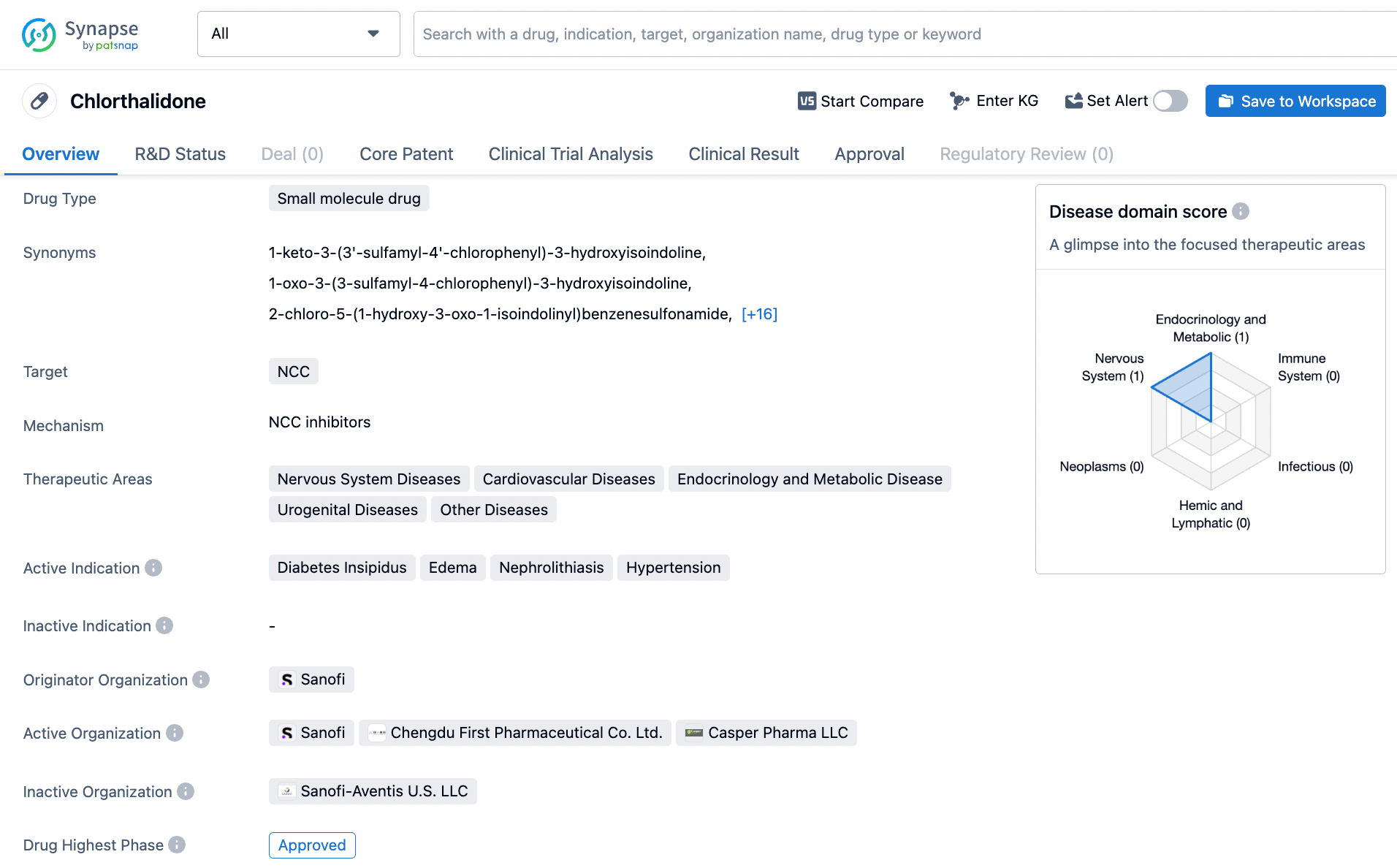
Strategically Search Chlorthalidone on Synapse: A How-to Guide
Chlorthalidone, a small molecule drug, operates via the inhibition of the NCC protein that is responsible for sodium and chloride reabsorption in the kidneys. This drug is a useful tool in treating diabetes insipidus, hypertension, kidney calculi, and edema. Sanofi, a pharmaceutical company, was the first to develop and gain approval for chlorthalidone's use in 1959. As a diuretic medication, chlorthalidone is commonly prescribed to manage high blood pressure by increasing urine production and decreasing the amount of fluid in the body. Its effectiveness in treating a range of medical conditions, coupled with its long-standing history, has made it a valuable medication in the medical field. Click on the image below to begin the exploration journey of Chlorthalidone through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!