What are GLP-1R agonists and how do you quickly get the latest development progress?
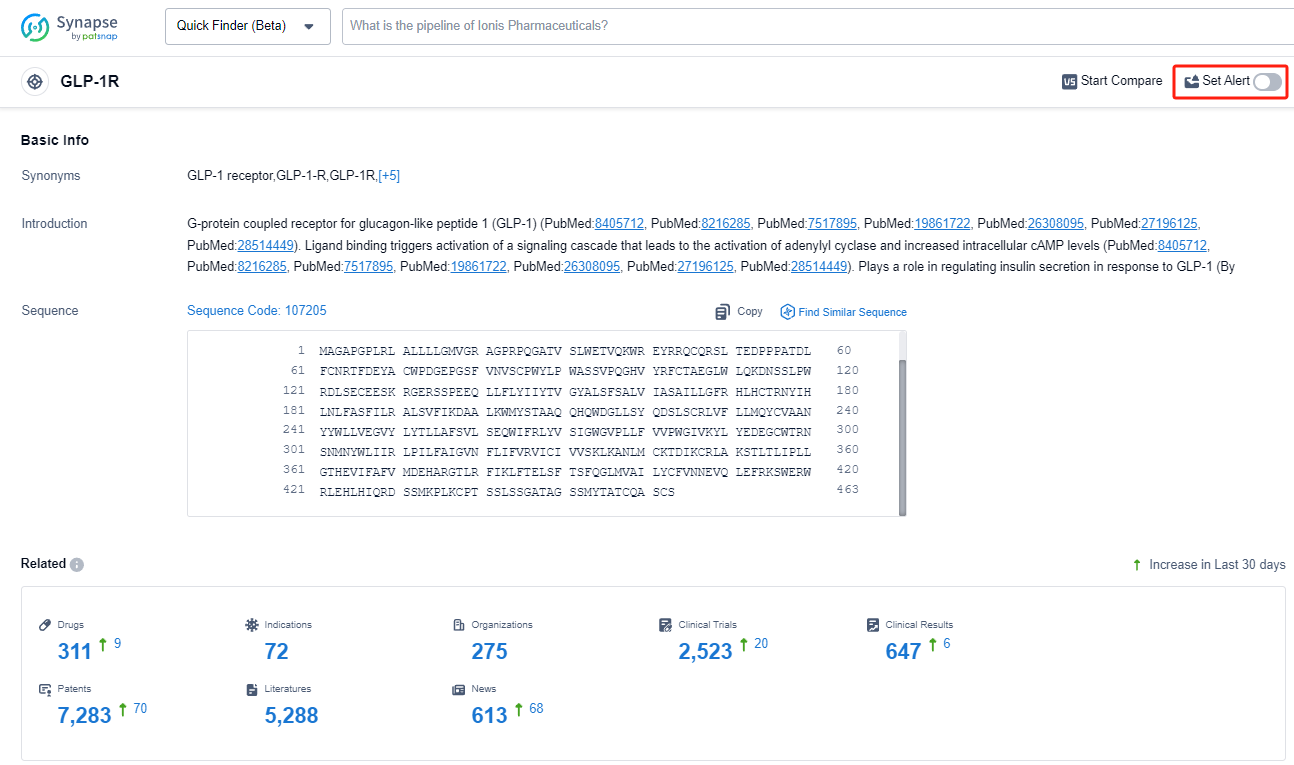
GLP-1R, or Glucagon-like peptide-1 receptor, plays a crucial role in the human body's regulation of glucose metabolism. It is primarily found in the pancreas, gastrointestinal tract, and central nervous system. When GLP-1 binds to its receptor, it stimulates insulin secretion, inhibits glucagon release, slows down gastric emptying, and promotes satiety. These actions help to regulate blood sugar levels, enhance glucose uptake, and control appetite. Due to its therapeutic potential, GLP-1R agonists have been developed as medications for the treatment of type 2 diabetes and obesity, offering a promising approach to managing these conditions.
The development of GLP-1 receptor agonists has been a significant milestone in the field of drug discovery. In 2005, Exendin-4 (Exenatide/ BYETTA), the world’s first GLP-1RA, was approved by the FDA for the adjuvant treatment of type 2 diabetes with two injections per day (BID). In 2009, Novo Nordisk’s Liraglutide (Victoza) was approved by the EMA and in January 2010 by the FDA for the treatment of type 2 diabetes.
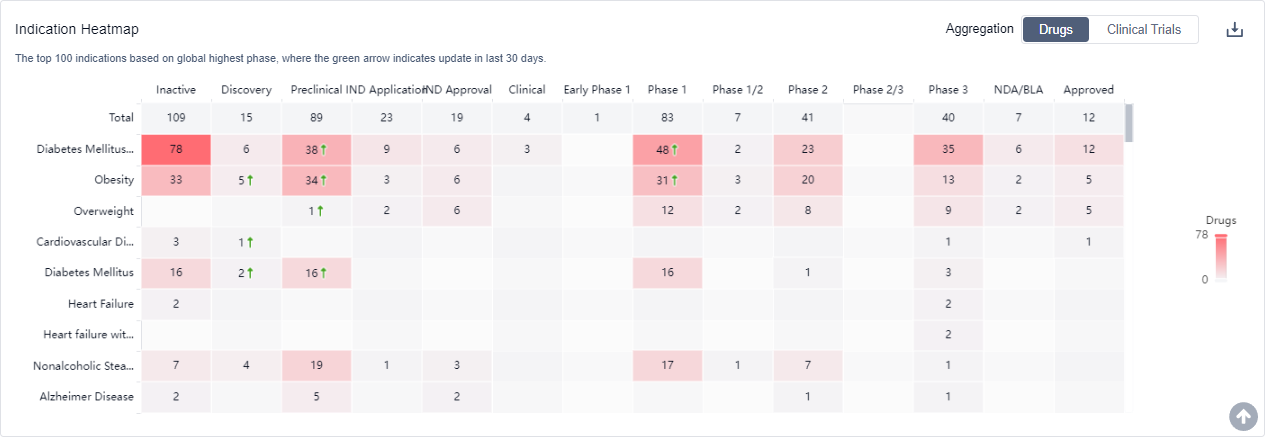
The analysis of the target GLP-1R reveals that Eli Lilly & Co., Novo Nordisk A/S, Sanofi, Huadong Medicine Co., Ltd., and AstraZeneca PLC are the companies growing fastest in this field. The main indication for drugs under this target is Diabetes Mellitus, Type 2. Synthetic peptide and Recombinant polypeptide are the drug types progressing most rapidly, indicating intense competition around the innovative drugs. China is the country with the highest development in this target, followed by the United States and Japan. The current competitive landscape of target GLP-1R is highly active, with multiple companies and drug types involved in the research and development of drugs for various indications. The future development of target GLP-1R is expected to continue at a rapid pace, with a focus on addressing the unmet needs in the treatment of Diabetes Mellitus, Type 2 and other related indications.
How do they work?
GLP-1R agonists are a type of medication used in the treatment of type 2 diabetes. GLP-1R stands for glucagon-like peptide-1 receptor, which is a receptor found on the surface of cells in the pancreas. Agonists are substances that bind to a receptor and activate it, causing a biological response.
In the context of biomedicine, GLP-1R agonists are designed to mimic the action of the hormone glucagon-like peptide-1 (GLP-1), which is naturally produced in the body. GLP-1 plays a role in regulating blood sugar levels by stimulating insulin release, reducing glucagon secretion, and slowing down the emptying of the stomach. By activating the GLP-1 receptor, GLP-1R agonists can enhance these effects and help improve blood sugar control in individuals with type 2 diabetes.
GLP-1R agonists are typically administered as injections and are available in various forms, including short-acting and long-acting formulations. They are often used as an adjunct to diet and exercise in the management of diabetes. These medications can help lower blood glucose levels, promote weight loss, and have been shown to have additional cardiovascular benefits.
It's important to note that GLP-1R agonists are not a cure for diabetes but rather a treatment option to help manage the condition. They may have side effects, including nausea, vomiting, and diarrhea, but these effects tend to improve over time. As with any medication, it is essential to consult with a healthcare professional for proper evaluation, prescription, and monitoring when considering the use of GLP-1R agonists.
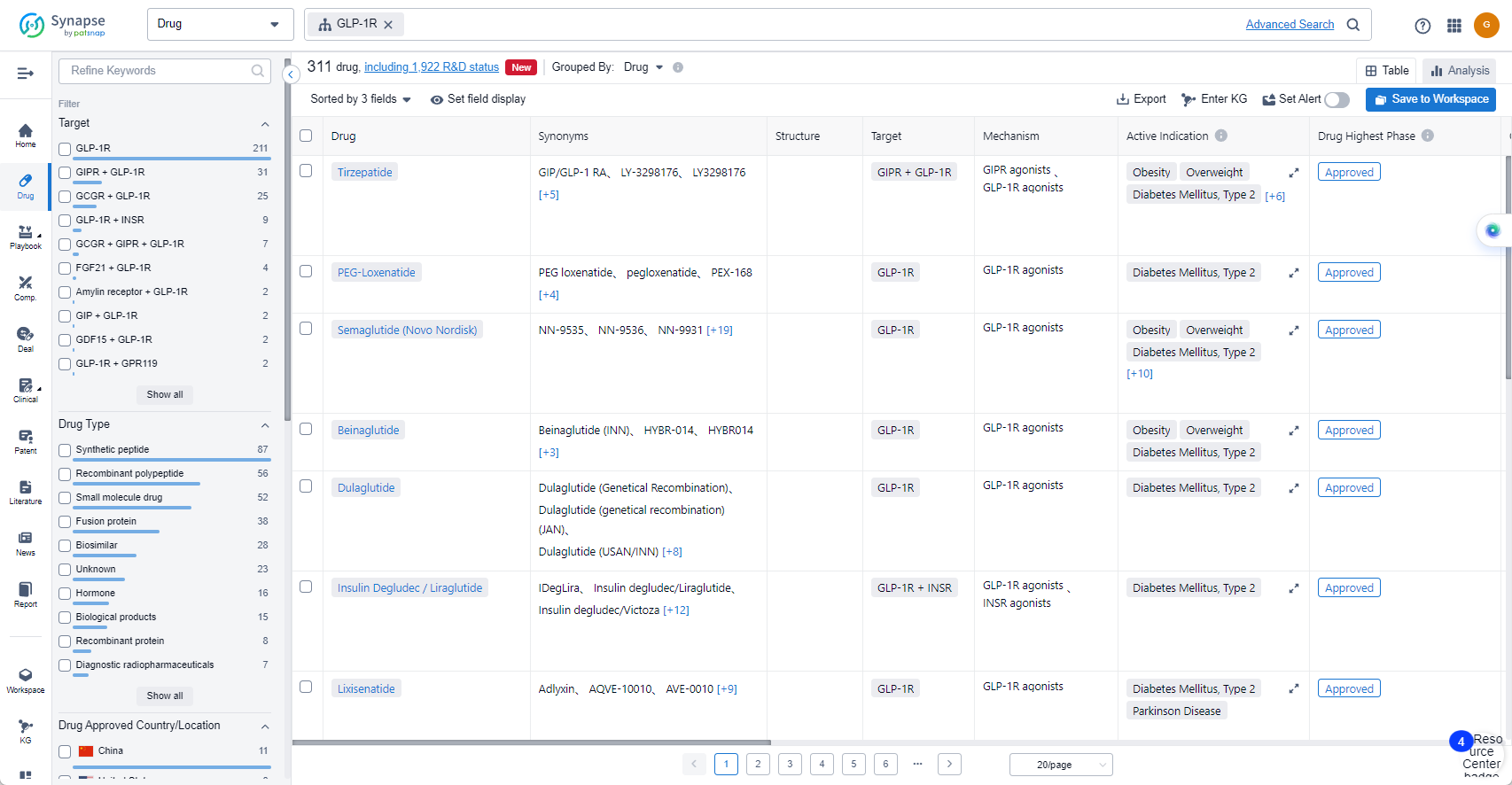
List of GLP-1R Agonists
The currently marketed GLP-1R agonists include:
- Tirzepatide
- PEG-Loxenatide
- Semaglutide (Novo Nordisk)
- Beinaglutide
- Dulaglutide
- Insulin Degludec / Liraglutide
- Lixisenatide
- Liraglutide Recombinant
- Exenatide
- Insulin Glargine/Lixisenatide
For more information, please click on the image below.
What are GLP-1R agonists used for?
GLP-1R agonists are used for the treatment of type 2 diabetes and obesity For more information, please click on the image below to log in and search.
How to obtain the latest development progress of GLP-1R agonists?
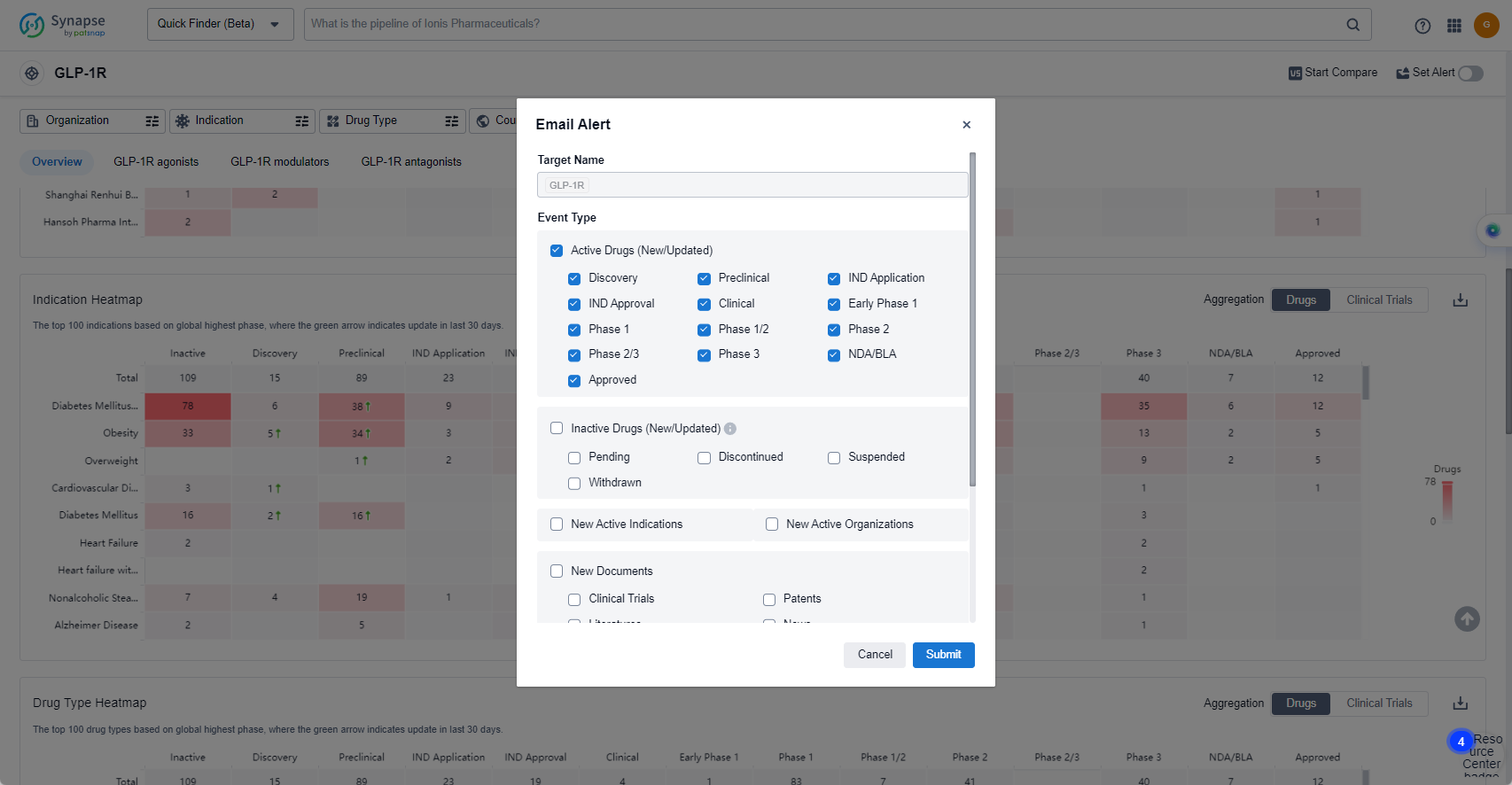
In the Synapse database, you can keep abreast of the latest research and development advances of GLP-1R agonists anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!