TransCode Therapeutics unveils extended lifespan in mouse models of Glioblastoma treated by its prime candidate, TTX-MC138
TransCode Therapeutics, Inc., an organization specializing in RNA oncology, dedicated to combating cancer through RNA treatment methods, today shared substantial enhancements in survival rates in mouse models with human glioblastoma multiforme tumors, post-treatment using their prime therapeutical candidate, TTX-MC138. The research unveiled that mice with implanted human GBM tumors and treated with TTX-MC138 exhibited noticeably extended survival in comparison to the control group subjects.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
GBM stands as the predominant and most virulent primary brain cancer within adults, posing the greatest death risk amid all brain tumors. In a research conducted by TransCode, a set of mice that had human GBM tumors inserted were subjected to TTX-MC138 treatment, serving as an alternate to the routine chemotherapy, temozolomide.
TMZ functions as the chief chemotherapy treatment for GBM. Yet, resistance to TMZ is prevalent in GBM cases and significantly contributes to the unsettling mortality rate tied to this illness. In this research, animals received either TTX-MC138 or TMZ via a systematic injection once a week for a six-week duration.
The lifespan of creatures treated with TTX-MC138 was noticeably longer than those of the control group. To be specific, 75% of mice treated with TTX-MC138 were still alive against 25% in the control group, 50 days post commencement of treatment.
Offering feedback on the research, TransCode’s CTO, Zdravka Medarova, PhD, stated, “We are of the belief that the encouraging results with TTX-MC138 hint at better survival rates for patients suffering from GBM. Crucially, we hold the view that TTX-MC138 might serve as an alternate solution in instances when resistance to routine chemotherapy comes into play.”
TTX-MC138 is structured of an iron oxide nanocarrier linked to a nucleic acid that's designed to inhibit cancer-causing RNA, microRNA-10b. MiRNA-10b is identified as the leading regulator of cancer progression in several developed solid tumors, GBM included.
TransCode suggests that TTX-MC138 could potentially serve as a cure for many of these types of cancer. Applying TTX-MC138 has resulted in the complete shrinkage of metastatic disease in several mouse models of pancreatic and breast cancer. Beyond mouse models of cancer, TTX-MC138 was effectively introduced and showed initial effectiveness in spontaneous feline mammary carcinoma.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
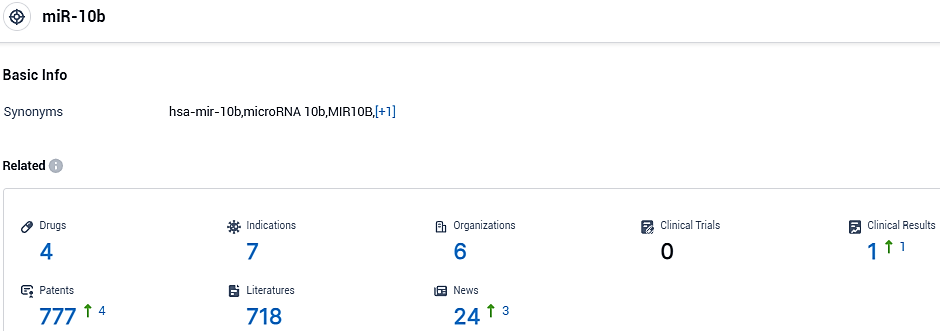
According to the data provided by the Synapse Database, As of October 31, 2023, there are 4 investigational drugs for the miR-10b target, including 7 indications, 6 R&D institutions involved,and as many as 777 patents.
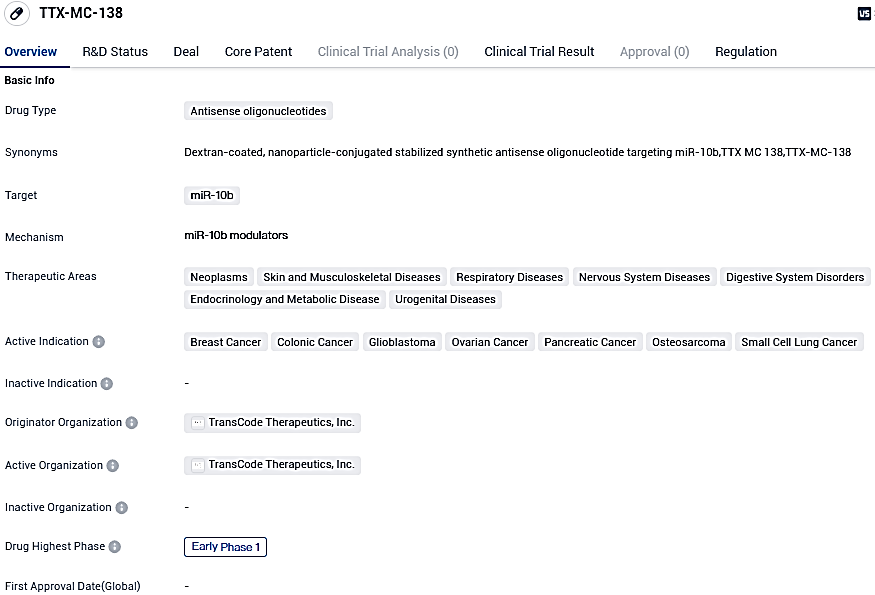
TTX-MC-138 is an antisense oligonucleotide medicine that aims at miR-10b, making it potentially effective for several types of cancerous conditions. It is presently in the Early Phase 1 of its development trail, indicating it as an orphan medicinal product, which shows its possible capability to tackle uncommon illnesses. Nevertheless, comprehensive evaluation of its efficacy and safety requires further research and clinical experiments.