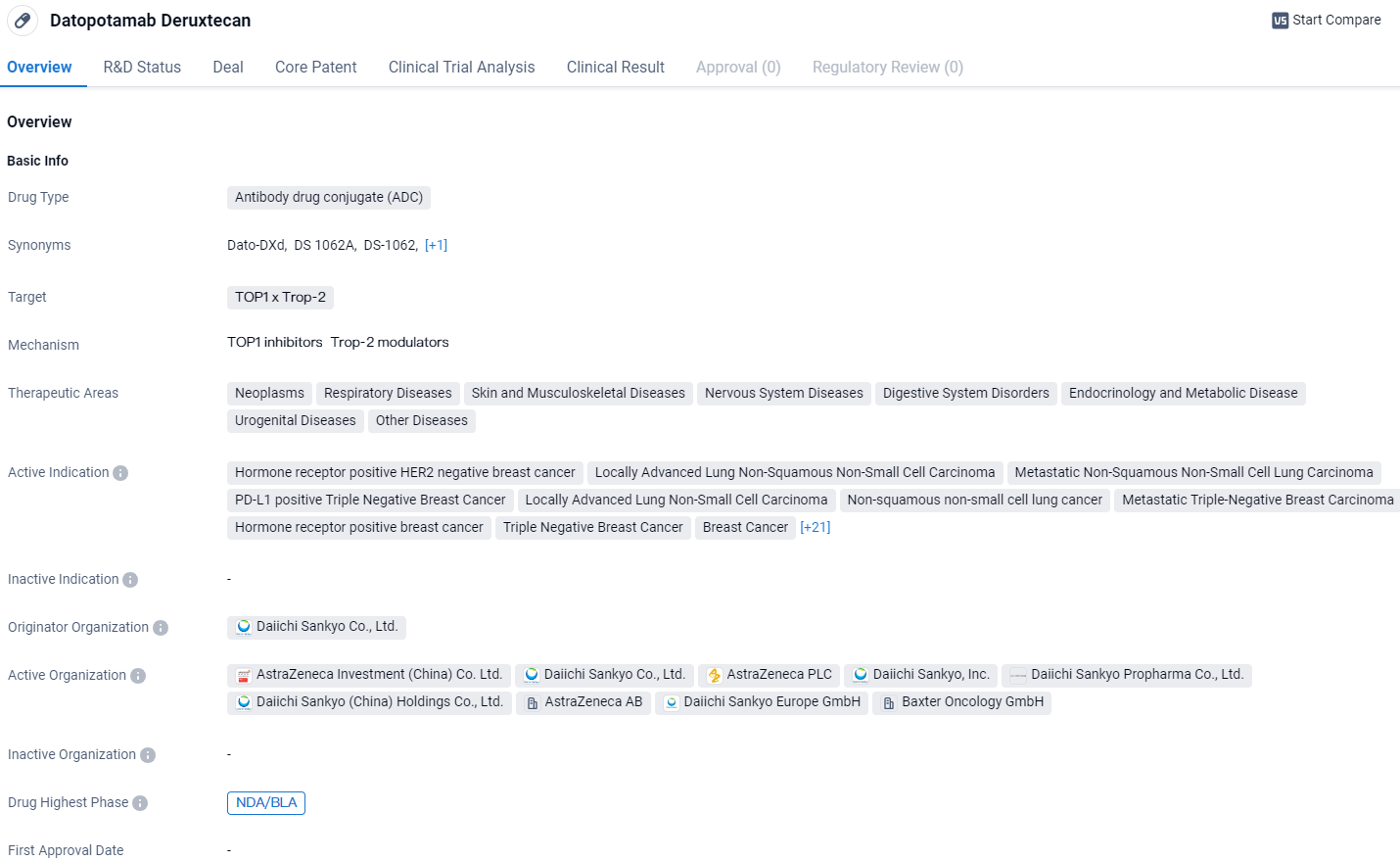
U.S. Review of Datopotamab Deruxtecan for Pre-Treated HR+/HER2- Breast Cancers
The U.S. Food and Drug Administration has acknowledged the submission from Daiichi Sankyo and AstraZeneca’s approval of datopotamab deruxtecan (Dato-DXd). This application seeks authorization to use the drug in treating adult individuals with advanced hormone receptor-positive, HER2-negative breast tumors who are no longer candidates for surgery or have cancer that has spread, and who have previously undergone systemic therapy for their advanced condition.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Developed by Daiichi Sankyo and co-developed with AstraZeneca, Datopotamab deruxtecan is a targeted therapy, specifically a TROP2 directed DXd antibody-drug conjugate. The anticipated date for the Prescription Drug User Fee Act (PDUFA) decision by the U.S. Food and Drug Administration on its approval is set for January 29, 2025.
The Biologics License Application (BLA) hinges on the outcomes of the critical TROPION-Breast01 phase 3 study. These results have been showcased at two significant events: the Presidential Symposium during the European Society for Medical Oncology Congress in 2023 and through an oral presentation at the San Antonio Breast Cancer Symposium the same year.
Datopotamab deruxtecan exhibited a significant and meaningful benefit in halting disease progression—a primary goal of the study—as opposed to the standard chemotherapy options chosen by the investigators. This was observed in patients with HR-positive, HER2-negative breast cancer who were not candidates for surgery or had metastatic disease, and had received hormone therapy and at least one line of systemic treatment prior.
While initial data indicated a potential advantage in overall survival for those treated with datopotamab deruxtecan compared to those receiving chemotherapy, these findings were not statistically conclusive at the time of the analysis. As the clinical trial presses forward, more comprehensive survival data will be gleaned from subsequent evaluations.
Susan Galbraith, MBBChir, PhD, Executive Vice President of Oncology R&D at AstraZeneca, emphasized the significance of this treatment advancement, noting that while there have been notable improvements in treating HR positive, HER2 negative breast cancer, those with advanced stages often build resistance to hormone therapy and may have to endure multiple chemotherapy regimens. She asserted that, upon receiving approval, datopotamab deruxtecan could offer these individuals an effective and less taxing substitute to traditional chemotherapy approaches.
Another BLA for this drug is also undergoing assessment in the USA. This application is based on data from the impactful TROPION-Lung01 phase 3 study, examining its efficacy for adults with advanced stages of nonsquamous non-small cell lung cancer who have had previous systemic treatments. Alongside this, further international regulatory filings for datopotamab deruxtecan addressing both lung and breast cancer are in progress.
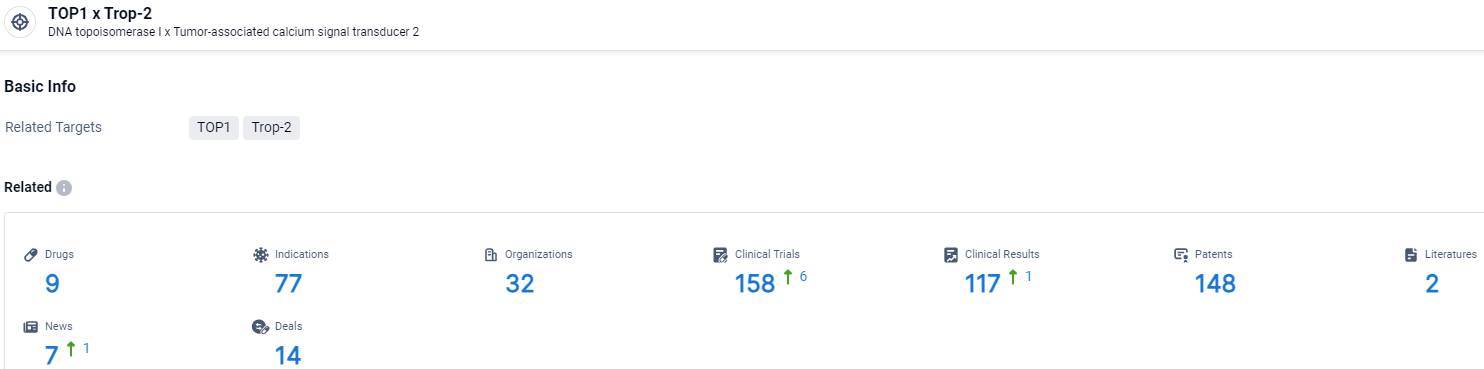
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 3, 2024, there are 9 investigational drugs for the TOP1 and Trop-2 target, including 77 indications, 32 R&D institutions involved, with related clinical trials reaching 158, and as many as 117 patents.
Datopotamab Deruxtecan targets TOP1 and Trop-2 and has shown promise in various therapeutic areas and indications. With its current status in the highest phase of development, the drug holds potential for treating a wide range of diseases and may offer improved efficacy and safety compared to traditional chemicals.