U.W. FDA notes Astellas' refiled biologics license for zolbetuximab, assigns new review date
Astellas Pharma Inc. revealed that the U.S. Food and Drug Administration has recognized their resubmission of the Biologics License Application for zolbetuximab. This is a pioneering investigational monoclonal antibody targeting claudin (CLDN) 18.2, intended for the first-line treatment of adults with locally advanced, unresectable, or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction adenocarcinoma, with tumors expressing CLDN18.2.
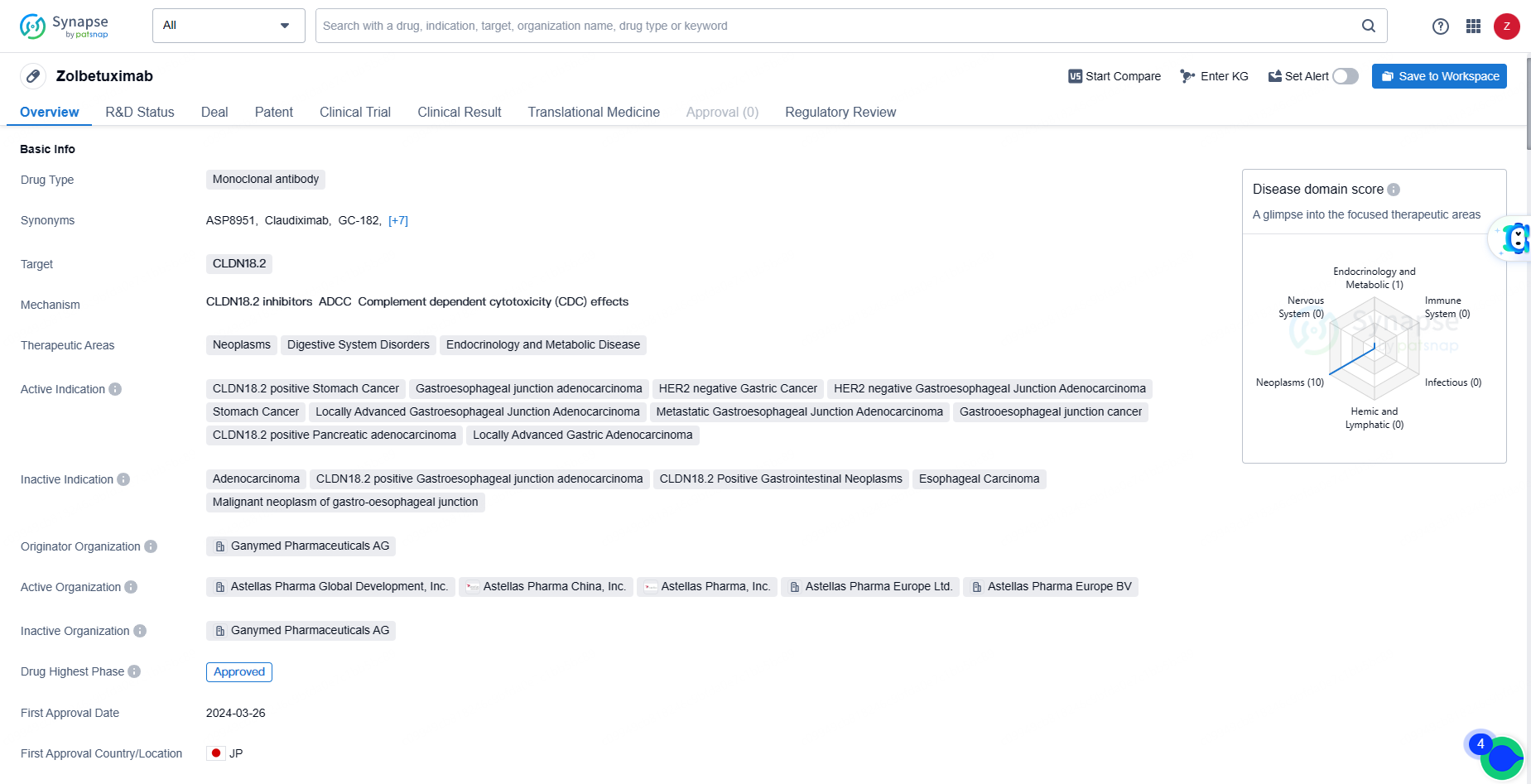
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Should it receive approval, zolbetuximab would become the first therapy targeting CLDN18.2 to be sanctioned for this patient group in the United States. The FDA has established a new target decision date under the Prescription Drug User Fee Act, which is November 9, 2024.
In the United States, projections for 2024 indicate that 26,890 individuals will receive a gastric cancer diagnosis, with 10,880 expected to succumb to the condition. Due to the overlap of early-stage gastric cancer symptoms with those of more prevalent stomach-related ailments, the disease is often detected at an advanced or metastatic stage, meaning it has spread from its initial site to other tissues or organs. The five-year relative survival rate for metastatic patients stands at 7%.
Moitreyee Chatterjee-Kishore, Ph.D., M.B.A., Senior Vice President and Head of Immuno-Oncology Development at Astellas, stated, "Astellas is dedicated to the development of novel targeted treatments for challenging cancers. Individuals suffering from advanced gastric or GEJ cancer frequently encounter significant unmet needs, and the FDA's recognition of the zolbetuximab BLA resubmission takes us a step closer to providing this crucial treatment option to suitable patients in the U.S. battling this deadly illness."
The resubmission of the zolbetuximab BLA occurred on May 9, 2024, in response to a complete response letter from the FDA on January 4, 2024, which cited deficiencies in third-party manufacturing discovered during a pre-licensure inspection. The FDA did not express any concerns regarding the clinical data's efficacy or safety and did not find additional clinical studies necessary to support the BLA approval.
The BLA for zolbetuximab was supported by data from the Phase 3 SPOTLIGHT and GLOW trials. The SPOTLIGHT trial compared zolbetuximab in combination with mFOLFOX6 (which includes oxaliplatin, leucovorin, and fluorouracil) to a placebo plus mFOLFOX6. Meanwhile, the GLOW trial examined zolbetuximab with CAPOX (a chemotherapy regimen of capecitabine and oxaliplatin) against a placebo plus CAPOX.
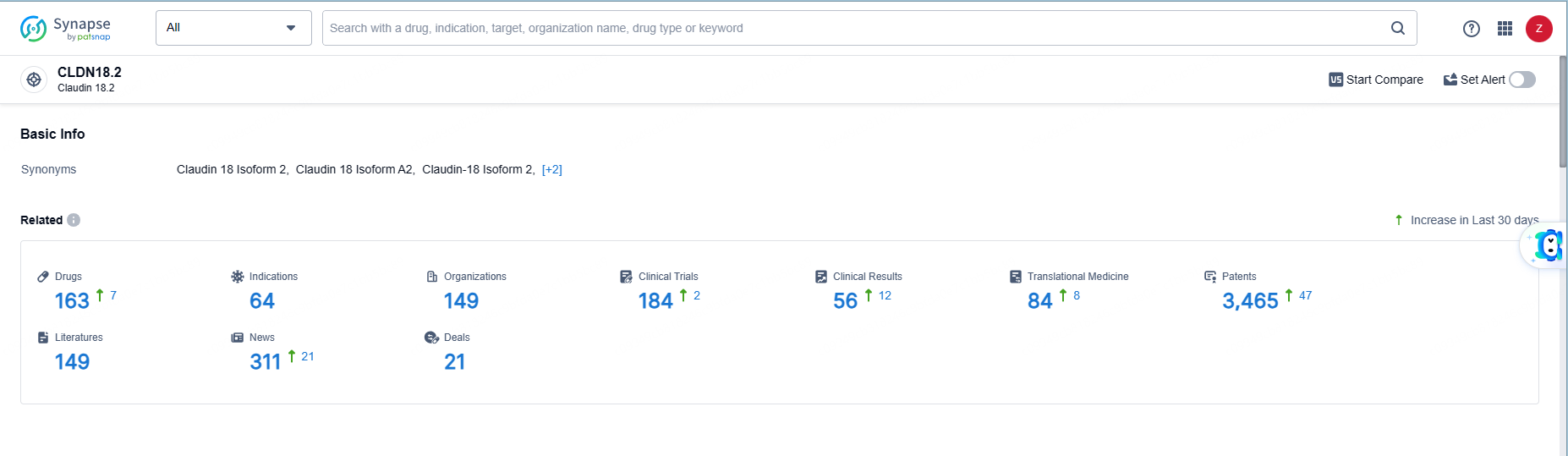
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 5, 2024, there are 163 investigational drugs for the CLDN18.2 target, including 64 indications, 149 R&D institutions involved, with related clinical trials reaching 184, and as many as 3465 patents.
As a monoclonal antibody targeting CLDN18.2, Zolbetuximab represents a novel approach to treating various cancers and digestive system disorders. Its approval in Japan and NDA/BLA status in China indicate the potential for global availability and accessibility of the drug in the near future.