What Does Canagliflozin Do?
Canagliflozin, marketed under the brand name Invokana, is a medication developed by Janssen Pharmaceuticals, a division of Johnson & Johnson, for the treatment of type 2 diabetes mellitus. It belongs to the drug class of SGLT-2 inhibitors, which work by inhibiting the sodium-glucose transport protein 2 (SGLT2), thereby reducing glucose absorption in the kidneys and lowering blood sugar levels. Canagliflozin is also indicated to lower the risk of cardiovascular events in adults with type 2 diabetes who have a history of heart disease and to reduce the risk of end-stage kidney disease in patients with type 2 diabetes-related kidney problems. It is not intended for the treatment of type 1 diabetes.
Mechanism of Action of Canagliflozin
Canagliflozin exerts its therapeutic effect by targeting the SGLT2 protein, which is responsible for the majority of renal glucose reabsorption. By inhibiting this protein, Canagliflozin increases the excretion of glucose in the urine, leading to lower blood glucose levels. This mechanism also promotes the excretion of calories, which can contribute to weight loss, an additional benefit for patients with type 2 diabetes.
How to Use Canagliflozin
Canagliflozin is available as an oral tablet with dosages of 100 mg and 300 mg. It is typically taken once per day, before the first meal of the day. The medication should be used as part of a comprehensive treatment plan that includes diet, exercise, weight control, blood sugar testing, and special medical care. Patients are advised to drink plenty of liquids while taking Canagliflozin and to follow their doctor's instructions closely regarding the management of their diabetes.
Side Effects of Canagliflozin
Canagliflozin can cause a range of side effects, some of which are serious. Common side effects include genital infections and increased urination. More serious side effects can include signs of an allergic reaction, symptoms of a genital infection, light-headedness, little or no urination, pain or burning when urinating, new pain or sores in the legs or feet, high potassium levels, ketoacidosis, and dehydration. Patients are advised to seek medical attention immediately if they experience these symptoms. Additionally, Canagliflozin may increase the risk of lower leg amputation, particularly in patients with pre-existing conditions such as foot ulcers, heart disease, or nerve damage.
Contraindications and Precautions
Canagliflozin is contraindicated in patients with severe kidney disease or those on dialysis. It should not be used during the second or third trimester of pregnancy and is not recommended for breastfeeding mothers. Patients with a history of heart problems, diabetic foot ulcers or amputations, circulation or nerve problems in the legs or feet, liver disease, bladder infections, or those who drink large amounts of alcohol should inform their doctor before taking Canagliflozin.
Drug Interactions with Canagliflozin
Canagliflozin may interact with other medications, including insulin, other diabetes medicines, diuretics, digoxin, rifampin, ritonavir, and seizure medications like phenobarbital and phenytoin. It is important to inform healthcare providers of all current medications and any changes to the medication regimen. Patients should also be aware that Canagliflozin can affect the results of certain medical tests and should inform any doctor treating them that they are taking the drug.
In conclusion, Canagliflozin is a valuable addition to the treatment options for adults with type 2 diabetes, offering a unique mechanism of action that can improve blood sugar control and reduce the risk of cardiovascular and kidney complications. However, due to its potential side effects and drug interactions, patients should be closely monitored by healthcare professionals and adhere to the prescribed treatment plan.
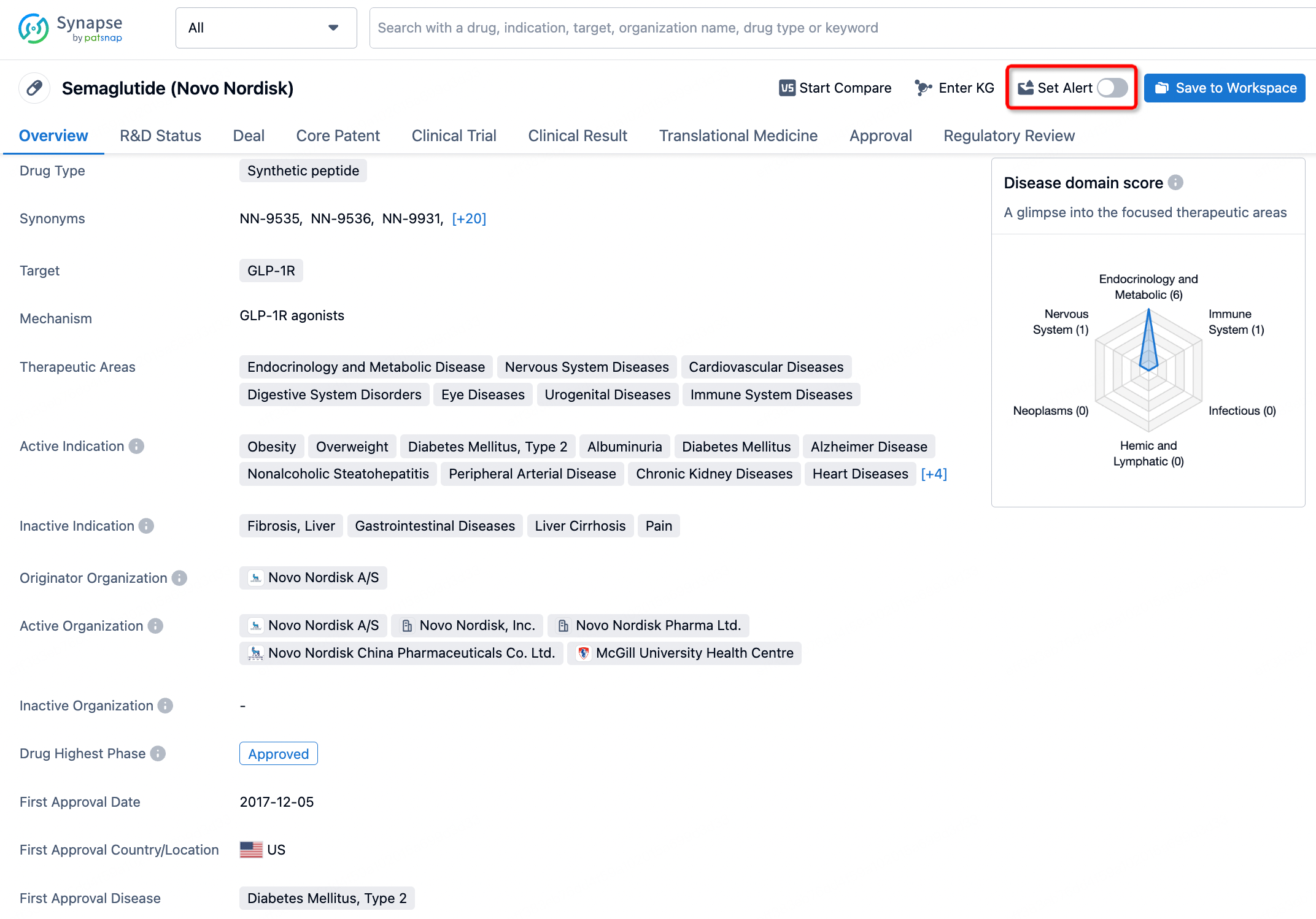
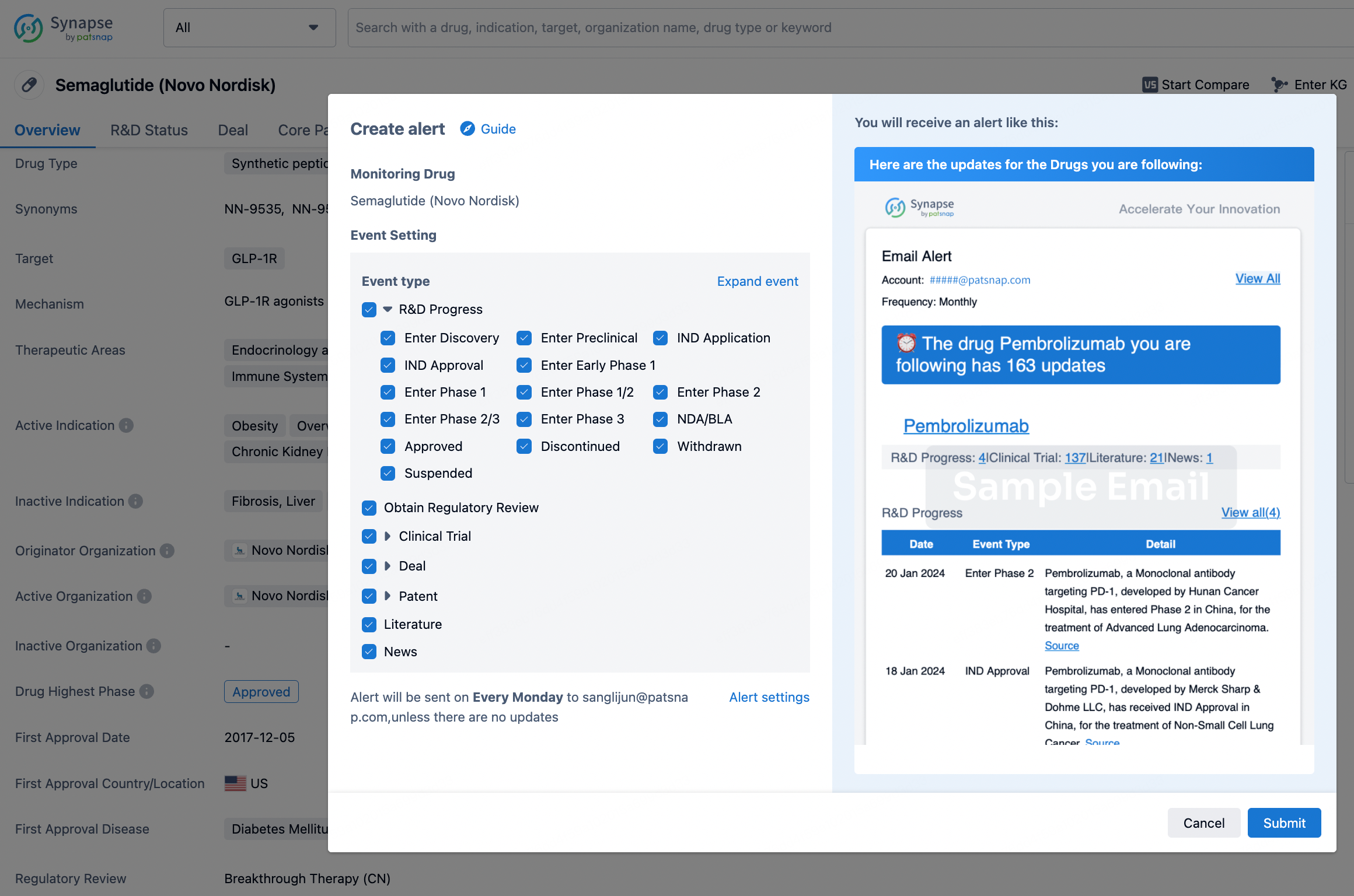
How to obtain the latest development progress of all drugs?
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!