Request Demo
Pharmaceutical Insights
Navigate pharmaceutical trends with our insights on targets, institutional pipelines, clinical advances, and new drugs.

Recent blog posts
Latest Hotspot
3 min read
Adicet Bio Showcases Preclinical Readiness of ADI-270 for IND at 27th ASGCT Meeting
25 April 2024
Adicet Bio presents preclinical findings indicating IND preparedness for ADI-270 during a talk at the 27th Annual ASGCT Meeting.
Pharma Frontiers
11 min read
Pharma Frontiers: Daily Digest of Global Pharmaceutical News - April 25
25 April 2024
April 25th latest updates in the global new drug development field, including progress in new drug research and development, transaction information, and partnership developments.
Latest Hotspot
3 min read
ASLAN Pharmaceuticals Announces Positive Early Results from Phase 2 Eblasakimab Trial in Atopic Dermatitis Patients Previously on Dupilumab
24 April 2024
ASLAN Pharmaceuticals Reports Encouraging Preliminary Outcomes from a Phase 2 Trial of Eblasakimab in Patients with Atopic Dermatitis Previously Treated with Dupilumab.
Graphic Pharma
19 min read
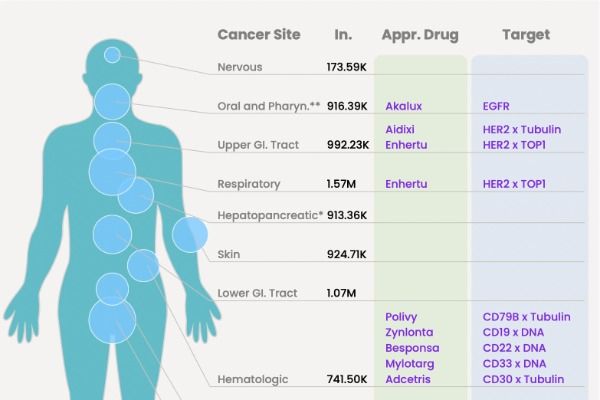
Compilation of 13 FDA-Approved ADC (Antibody-Drug Conjugate) Medications
24 April 2024
At present, 13 types of ADCs have been approved by the United States Food and Drug Administration (FDA), with over 200 additional ADCs at various stages of clinical trials.
Latest Hotspot
3 min read
AGA China Phase II Study GT20029 Successfully Meets Its Primary Goal
24 April 2024
Kintor Pharmaceutical Limited announced that its innovative proteolysis targeting chimera compound, GT20029, targeting the androgen receptor (AR), has achieved the primary endpoint in a phase II trial in China.
"What" Series
2 min read
What is ED50?
24 April 2024
ED50 (Effective Dose 50) refers to the dose of a drug or substance that produces a desired effect in 50% of a population or sample under a given set of experimental conditions or in a group of test subjects.
Latest Hotspot
3 min read
EU Commission Approves BIMZELX® for Hidradenitis Suppurativa, First Biologic Targeting IL-17A and IL-17F
24 April 2024
The European Commission has granted UCB approval for BIMZELX®(bimekizumab), marking it as the initial biologic targeting both IL-17A and IL-17F for treating moderate to severe hidradenitis suppurativa.
Hot Spotlight
7 min read
Tavapadon: A New Hope in the Battle Against Parkinson's Disease
24 April 2024
The challenge is finding how to effectively alleviate these symptoms while maintaining sufficient safety. Tavapadon has found a balance in addressing this issue.
Latest Hotspot
3 min read
FDA Approves Takeda's ENTYVIO® for Crohn’s Disease Treatment
24 April 2024
U.S. FDA Sanctions Takeda’s ENTYVIO® (vedolizumab) for Subcutaneous Use in Managing Moderate to Severe Crohn’s Disease.
"What" Series
2 min read
What is the mechanism of action of radioactive therapeutic drugs?
24 April 2024
Radioactive therapeutic drugs, also known as radioactive drugs, mainly function based on the particles or rays produced by the radioactive decay of radionuclides.
Latest Hotspot
3 min read
Chugai In-Licenses RNAi Therapeutic Zilebesiran for Hypertension with High Cardiovascular Risk
24 April 2024
Chugai Secures Licensing Deal with Roche for Hypertension Drug Zilebesiran in Japan.
Pharma Frontiers
13 min read
Pharma Frontiers: Daily Digest of Global Pharmaceutical News - April 24
24 April 2024
April 24th latest updates in the global new drug development field, including progress in new drug research and development, transaction information, and partnership developments.