Request Demo
Pharmaceutical Insights
Navigate pharmaceutical trends with our insights on targets, institutional pipelines, clinical advances, and new drugs.

Recent blog posts
Pharma Pioneer
8 min read
Stop Drowning in Data: Meet Synapse Lens, the Ultimate Reading Assistant for Biomed Professionals
5 February 2026
If you’ve ever felt overwhelmed by the sheer volume of reading required to stay ahead, there is a tool designed specifically for you. Introducing Synapse Lens, a Chrome extension hailed as a "superpower" for pharmaceutical and biotech professionals.
Pharma Frontiers
8 min read
Aqvesme’s FDA Milestone: Expanding Mitapivat’s Reach in Thalassemia Treatment
22 January 2026
The FDA’s recent approval of Aqvesme (mitapivat) for the treatment of anemia in adults with alpha- and beta-thalassemia marks a significant expansion for pyruvate kinase (PK) activators. By enhancing the activity of the wild-type PK enzyme, Aqvesme improves red blood cell health and hemoglobin levels, offering a potent oral alternative to chronic transfusions for patients regardless of their specific genetic mutation. This broad indication underscores a major shift in metabolic regulation therapy, requiring R&D teams to leverage deep structural intelligence to map the competitive landscape of PK activators and identify future modification opportunities for next-generation erythroid therapies.
Pharma Frontiers
10 min read
The $4B Patent Cliff: Navigating the Generic Surge as Astellas’ Xtandi Nears Expiration
22 January 2026
Astellas Pharma is facing a defining moment as its blockbuster prostate cancer therapy, Xtandi (enzalutamide), approaches a massive patent cliff in 2026 and 2027. With billions in annual revenue at risk, the pharmaceutical giant is pivoting toward aggressive lifecycle management and strategic BD (Business Development) to offset the impending generic competition. For generic manufacturers and oncology innovators, this transition creates a high-stakes environment where the ability to rapidly deconstruct original patent claims and identify "late-stage" IP vulnerabilities is the key to capturing market share in a post-Xtandi era.
Latest Hotspot
10 min read
Beyond the Needle: Eli Lilly’s Orforglipron and the Small Molecule GLP-1 Revolution
21 January 2026
The weight loss market is undergoing a seismic shift as the focus moves from injectable peptides to oral small molecule GLP-1 receptor agonists, led by Eli Lilly’s promising candidate, orforglipron. By overcoming the historical bioavailability challenges of traditional peptides, orforglipron offers a highly potent, non-peptide alternative that could drastically lower production costs and improve patient adherence. This evolution from biologic to chemical synthesis highlights a critical need for advanced SAR (Structure-Activity Relationship) mapping, as researchers race to optimize these non-peptide scaffolds for maximum potency and minimal side effects in a hyper-competitive global landscape.
Latest Hotspot
20 min read
A Historic First for Menkes Disease: How Zycubo’s FDA Approval is Redefining Rare Disease R&D
21 January 2026
The FDA’s landmark approval of Zycubo (copper histidinate) on January 13, 2026, marks a historic breakthrough as the first-ever therapy for Menkes disease, a rare and previously fatal neurodegenerative disorder. By delivering a stabilized copper-histidine complex that effectively bypasses ATP7A-related genetic defects, Zycubo has demonstrated a staggering 80% reduction in mortality risk for early-intervention cohorts, extending median overall survival from 17.6 months to an unprecedented 177.1 months. This success story underscores the critical role of high-precision molecular intelligence in orphan drug R&D, illustrating how advanced data mining can unlock life-saving potential within the most complex chemical and patent landscapes.
Drug Insights
6 min read
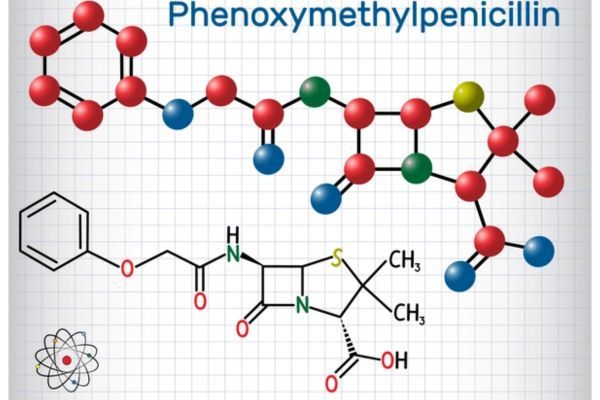
Market Analysis of Phenoxymethylpenicillin (Penicillin V) in the United States
5 September 2025
Discover the U.S. market dynamics of phenoxymethylpenicillin (Penicillin V), including FDA approvals, clinical research, patent analysis, regulatory risks, and opportunities for generic market entry.
Drug Insights
6 min read
Market Analysis of Neostigmine Methylsulfate in the United States
5 September 2025
Explore the U.S. market landscape of neostigmine methylsulfate, including FDA approvals, patent analysis, clinical data, regulatory risks, and opportunities for differentiation in a competitive generic market.
Drug Insights
6 min read
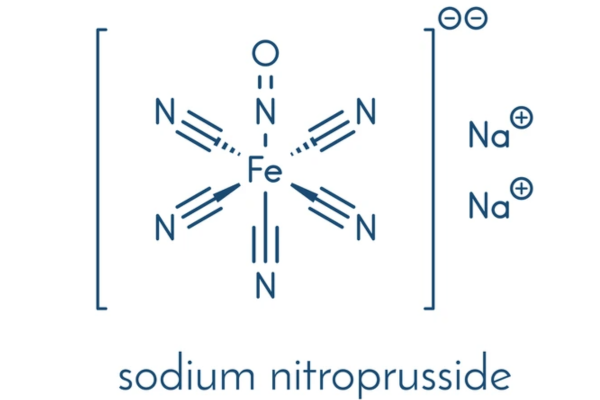
Market Analysis of Sodium Nitroprusside in the USA: Patent Landscape
5 September 2025
Explore the US market analysis of Sodium Nitroprusside, including approvals, patent barriers, clinical results, and strategies for generic competition and product differentiation.
Drug Insights
6 min read
Market Analysis of Droperidol in the USA: Regulatory History, Safety Risks, and Reintroduction Pathways
4 September 2025
Market analysis of Droperidol in the USA — overview of approvals, safety (QT prolongation), patent status, and strategic recommendations for potential reintroduction.
Drug Insights
6 min read
Market Analysis of Etoricoxib in the USA: Regulatory Barriers and Market Opportunities
4 September 2025
Etoricoxib, a selective COX-2 inhibitor by Merck, is widely approved outside the USA but remains unapproved domestically. Explore its global presence, patent landscape, clinical data, and regulatory challenges in the US market.
Drug Insights
6 min read
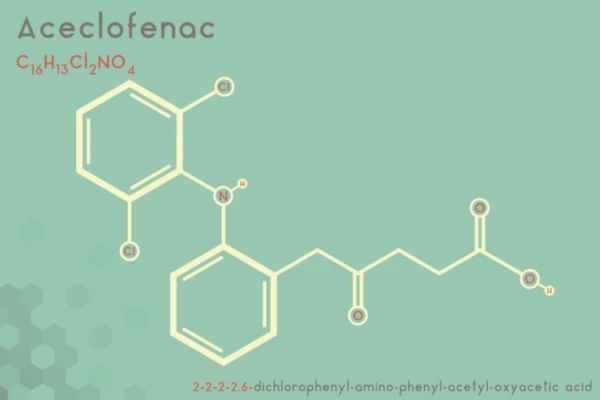
Market Analysis of Aceclofenac in the USA: Opportunities and Barriers for Market Entry
29 August 2025
Aceclofenac is a widely used NSAID in Europe and China but remains unapproved in the USA. Explore its global approvals, patent landscape, clinical results, and strategies for US market entry.
Drug Insights
6 min read
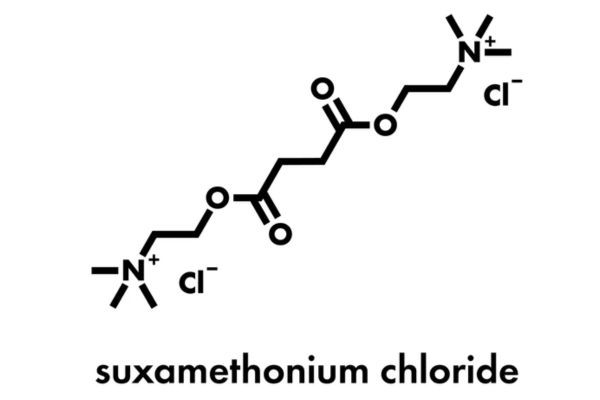
Market Analysis of Succinylcholine Chloride in the USA: Opportunities and Patent Landscape
29 August 2025
Succinylcholine Chloride has been in the US market since 1952 as a key anesthetic agent. Explore its approvals, clinical results, patent barriers, and strategies for market entry and innovation.