A Comprehensive Review of Umeclidinium Bromide's R&D Innovations
Umeclidinium Bromide's R&D Progress
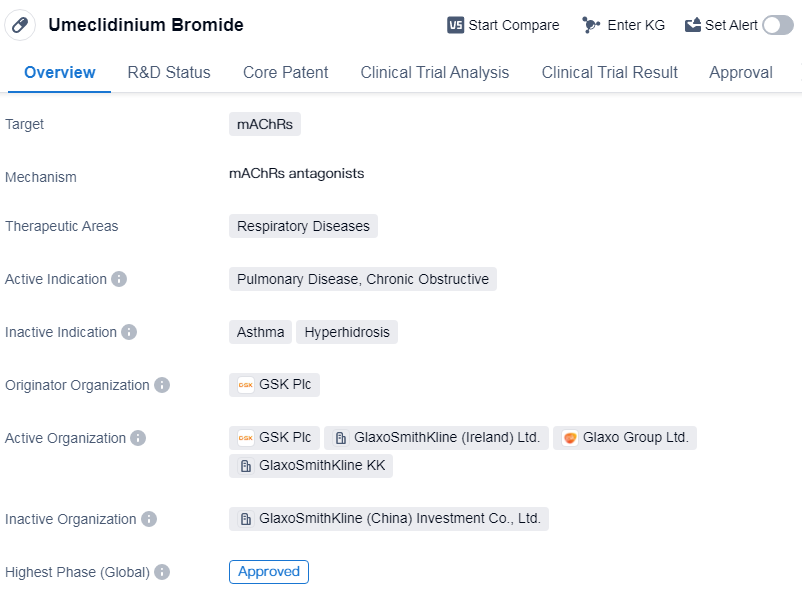
Umeclidinium Bromide is a small molecule drug that falls under the therapeutic area of respiratory diseases. It specifically targets muscarinic acetylcholine receptors (mAChRs) and is primarily indicated for the treatment of pulmonary disease, chronic obstructive. The drug was first approved globally in Iceland in February 2014.
The originator organization of Umeclidinium Bromide is GSK Plc, a renowned pharmaceutical company. The drug has reached the highest phase of approval globally, indicating its successful development and regulatory clearance in these regions.
Being a small molecule drug, Umeclidinium Bromide is designed to interact with specific receptors in the body, in this case, mAChRs. These receptors are found in the smooth muscles of the airways and play a crucial role in regulating bronchoconstriction. By targeting mAChRs, Umeclidinium Bromide aims to alleviate the symptoms associated with chronic obstructive pulmonary disease (COPD), a progressive lung disease characterized by airflow limitation and persistent respiratory symptoms.
The approval of Umeclidinium Bromide in Iceland in 2014 signifies its safety and efficacy profile, as assessed by regulatory authorities. This milestone allows healthcare professionals in Iceland to prescribe the drug to patients suffering from COPD, providing them with a potential treatment option to manage their condition.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Umeclidinium Bromide: mAChRs antagonists
mAChRs antagonists refers to muscarinic acetylcholine receptor antagonists. Muscarinic acetylcholine receptors (mAChRs) are a type of G protein-coupled receptors found in the central and peripheral nervous systems. They are activated by the neurotransmitter acetylcholine and play a crucial role in various physiological processes.
Antagonists, in the context of mAChRs, are substances that bind to these receptors without activating them. Instead, they block the binding of acetylcholine to the receptors, thereby inhibiting their normal function. mAChRs antagonists are commonly used in medicine to treat conditions such as overactive bladder, chronic obstructive pulmonary disease (COPD), and certain types of gastrointestinal disorders.
By blocking the activation of mAChRs, antagonists can help reduce excessive cholinergic activity, which can lead to symptoms such as increased smooth muscle contraction, excessive glandular secretions, and abnormal neuronal signaling. These drugs can provide therapeutic benefits by selectively inhibiting mAChRs in specific tissues or organs, leading to the desired pharmacological effects.
It's important to note that mAChRs antagonists can have various subtypes and selectivity profiles, targeting different mAChR subtypes (M1-M5) with varying affinities. The specific subtype selectivity of an antagonist can determine its therapeutic effects and potential side effects.
Drug Target R&D Trends for Umeclidinium Bromide
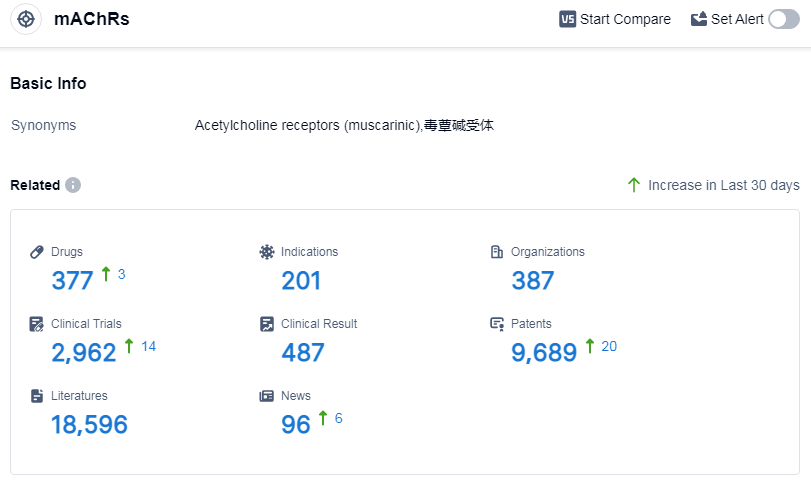
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 377 mAChRs drugs worldwide, from 387 organizations, covering 201 indications, and conducting 2962 clinical trials.
The analysis of target mAChRs reveals a competitive landscape with multiple companies actively involved in research and development. Pfizer Inc. leads in terms of the highest stage of development, with several approved drugs and ongoing R&D efforts. Indications such as pulmonary disease, chronic obstructive, urinary bladder, overactive, and spasm have a significant number of approved drugs, indicating their therapeutic relevance. Small molecule drugs dominate the drug types, with biosimilars like monoclonal antibodies also present. China, Japan, the United States, and the European Union are the key countries/locations driving development under the target mAChRs.
Overall, the target mAChRs present opportunities for further research and development, with potential for innovative drugs and competition in the pharmaceutical industry. The findings of this analysis can guide strategic decision-making and future development in this field.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Umeclidinium Bromide is a small molecule drug that developed by GSK Plc. The drug's first approve took place in Iceland and it has reached the highest phase of approval globally, which indicates its successful development and regulatory clearance.