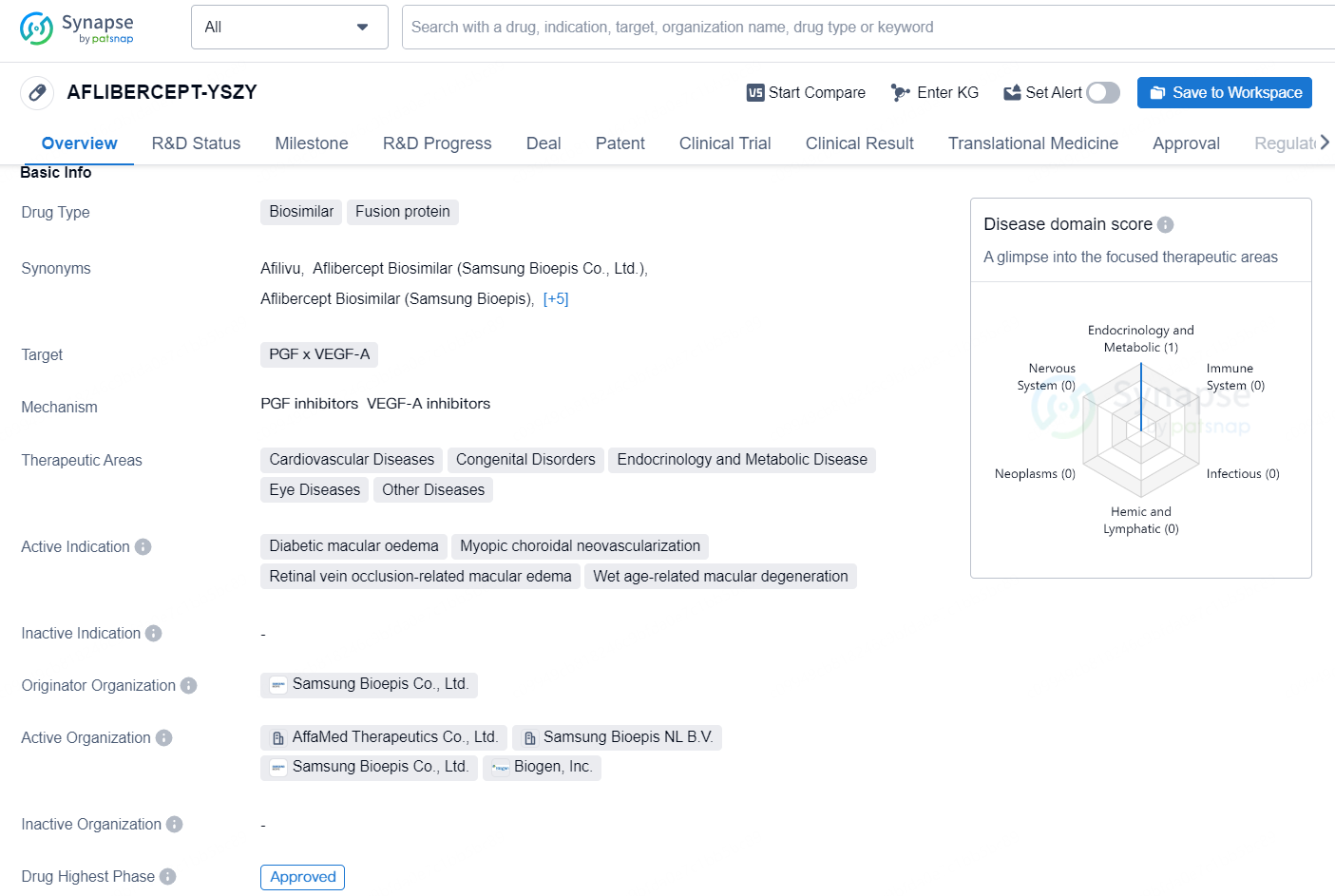
EC Approves Samsung Bioepis and Biogen's OPUVIZ Aflibercept Biosimilar
Samsung Bioepis Co., Ltd. in collaboration with Biogen Inc. (Nasdaq: BIIB) has announced that the European Commission (EC) has granted approval for OPUVIZ™ 40 mg/mL injectable solution in a vial. This biosimilar is based on Eylea (aflibercept) and was developed and registered by Samsung Bioepis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
OPUVIZ, referred to as SB15, has received approval for use in adult patients for the management of neovascular (wet) age-related macular degeneration (AMD), visual impairment stemming from macular edema linked to retinal vein occlusion (RVO; either branch or central), visual impairment caused by diabetic macular edema (DME), and visual impairment from myopic choroidal neovascularization (myopic CNV).
Byoungin Jung, Vice President of Regulatory Affairs at Samsung Bioepis, stated, “Retinal diseases impact the lives of millions in Europe, yet numerous patients encounter obstacles to treatment due to exorbitant costs. The European Commission’s approval for OPUVIZ is a significant advancement toward our objective of increasing access to essential biologic therapies for those who require them. We remain committed to broadening the availability of our high-quality, safe, and effective biosimilars to enhance patients’ quality of life and ensure the sustainability of healthcare systems.”
Wolfram Schmidt, Head of Europe at Biogen, commented, “This approval marks an exciting development for both patients and healthcare providers in Europe. OPUVIZ could introduce a new treatment alternative for eligible individuals, while also helping to mitigate the financial burden associated with these retinal disorders. We are pleased to continue our collaboration with Samsung Bioepis, with this being the approval of our fifth biosimilar treatment in Europe.”
The approval by the European Commission was founded on a comprehensive body of evidence, which encompassed analytical, non-clinical, and clinical data. A Phase 3 study that was randomized, double-masked, parallel-group, and multicenter showed that SB15 achieved similar efficacy and comparable safety, immunogenicity, and pharmacokinetics (PK) profiles when compared to the reference drug aflibercept (AFL). The main endpoint was achieved regarding the change from baseline in best corrected visual acuity (BCVA) at week 8, and analyses at 32 weeks and 56 weeks indicated comparable results for other secondary efficacy, safety, immunogenicity, and PK endpoints between SB15 and AFL.
OPUVIZ is the second biosimilar approved in the field of ophthalmology in Europe and the fifth in the range developed by Samsung Bioepis, with Biogen holding commercialization rights. This portfolio includes BYOOVIZ™ (ranibizumab), BENEPALI™ (etanercept), IMRALDI™ (adalimumab), and FLIXABI™ (infliximab). In November 2019, a commercialization agreement was established between Samsung Bioepis and Biogen for two ophthalmology biosimilar candidates, BYOOVIZ (SB11, ranibizumab) and OPUVIZ (SB15, aflibercept), across the U.S., Canada, Europe, and several other markets.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
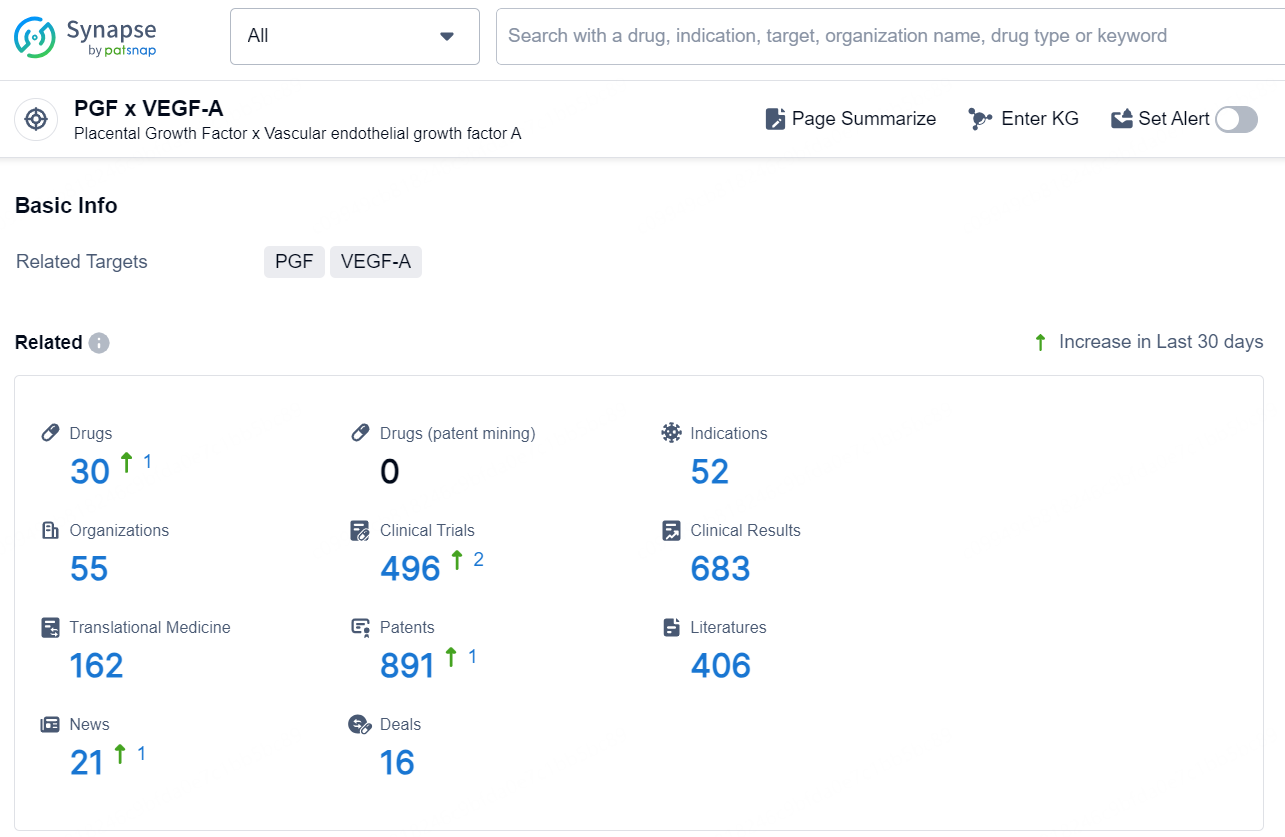
According to the data provided by the Synapse Database, As of November 19, 2024, there are 30 investigational drugs for the PGF x VEGF-A target, including 52 indications, 55 R&D institutions involved, with related clinical trials reaching 496, and as many as 891 patents.
AFLIBERCEPT-YSZY is a biosimilar fusion protein drug that targets PGF x VEGF-A. It is indicated for various therapeutic areas including cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, eye diseases, and other diseases.