Edgewise Therapeutics Launches Worldwide Crucial Trial of EDG-5506 for Becker Muscular Dystrophy, Called GRAND CANYON
As a reputable biopharmaceutical corporation focused on muscular disorders, Edgewise Therapeutics, Inc., has initiated the participation induction for GRAND CANYON, an international principal examination of EDG-5506 designed for Becker patients. GRAND CANYON originates from an extension of the CANYON research. CANYON's enrollees exceeded its original target, it contains 39 mature participants and 24 teenage participants.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
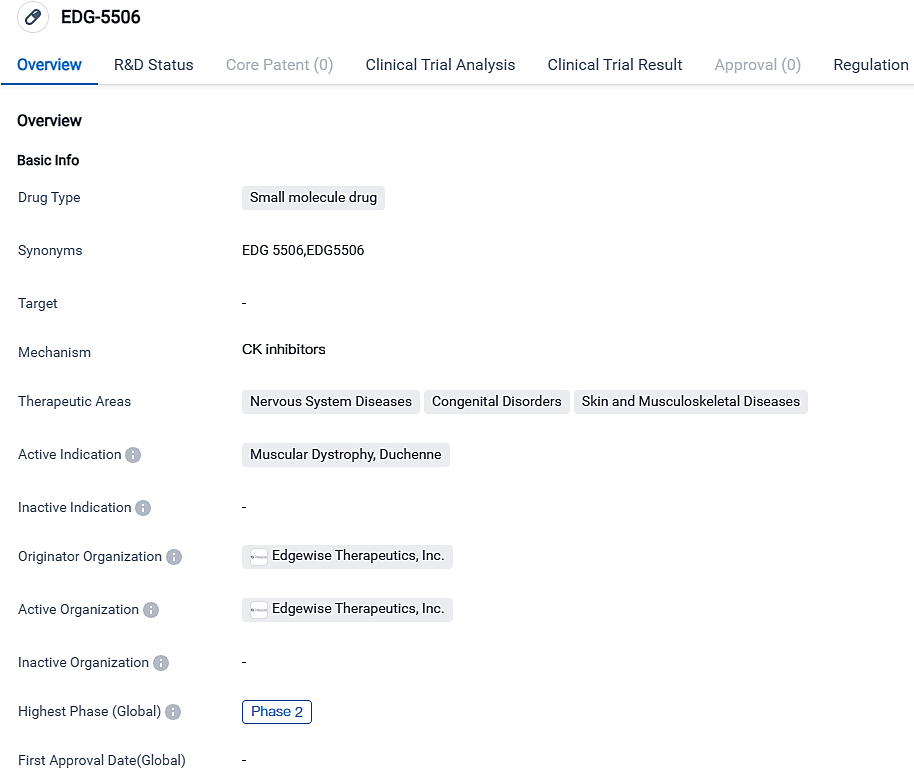
EDG-5506 serves as a small molecule that, when taken orally, is engineered to hinder contraction-related muscular destruction tied to dystrophinopathies, encompassing Becker and Duchenne muscular dystrophy. At present, there are no approved treatments for Becker, a severe genetically progressive neuromuscular abnormality with substantial unmet requirements.
The multicenter, randomized, double-blinded, placebo-controlled investigation, referred to as GRAND CANYON, aims to assess the safety and efficiency of EDG-5506 in adults with Becker. The GRAND CANYON could provide support for a marketing application if the data obtained is positive. The essential outcome of the GRAND CANYON is the North Star Ambulatory Assessment.
Furthermore, other functional evaluations and muscle damage biomarkers, along with safety, will be gauged. The estimated recruitment number for GRAND CANYON is around 120 Becker sufferers, aged from 18 to 50 years, occurring in approximately 50 locations across 10 countries. The participant's treatment duration is set to be 18 months.
"Relying on the robustness of safety, viable, and biomarker results obtained from our ARCH open-label investigation, we've promptly initiated this milestone clinical study," cited Joanne Donovan, M.D., Ph.D., the Chief Medical Officer at Edgewise. "This study plays a crucial role for the Becker population, seeing that there are currently no sanctioned treatments for Becker, a condition that has been neglected for an extended period."
"Transitioning to the next level of clinical speculation testing is thrilling, primarily when the hypothesis suggests that a decrease in contraction-caused muscle damage could be advantageous to individuals battling with muscular dystrophies," expressed Alan Russell, Ph.D., the Co-Founder and Chief Scientific Officer at Edgewise. "I am delighted to enter a pivotal study as we've observed promising preclinical outcomes with EDG-5506 translating to a clinical level. "
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
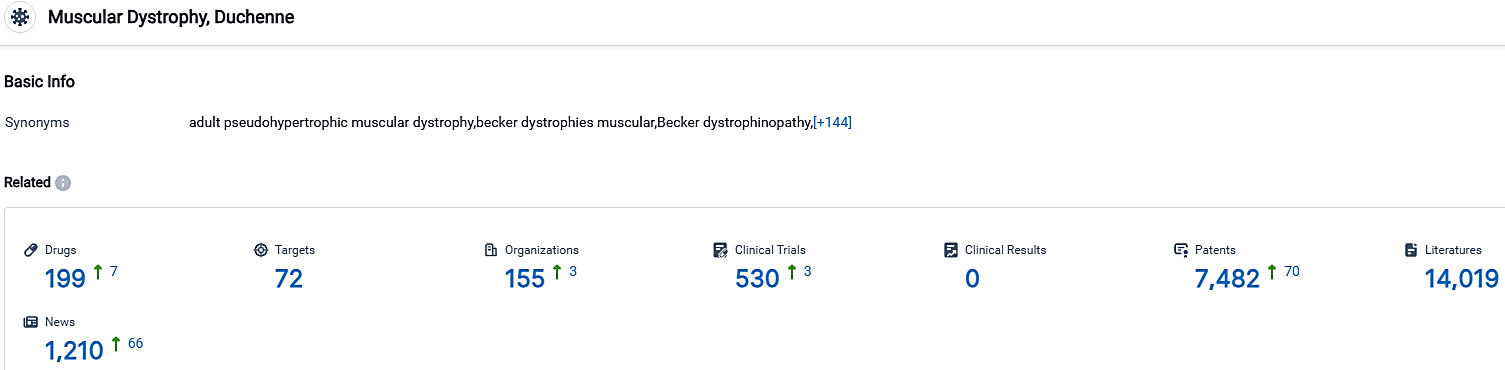
According to the data provided by the Synapse Database, As of October 2, 2023, there are 199 investigational drugs for the Muscular Dystrophy, including 72 targets 155 R&D institutions involved, with related clinical trials reaching 530,and as many as 7482 patents.
EDG-5506 is showing considerable promise as a potential treatment for Muscular Dystrophy, particularly Duchenne's variant. Its progress to Phase 2 clinical tests and the assignment of a Fast Track status suggest that it exhibited favorable results during preliminary phases of research. Nonetheless, additional clinical examinations and official authorizations will be required to establish its safe usage and effectiveness.