Exploring Sulodexide's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Sulodexide's R&D Progress
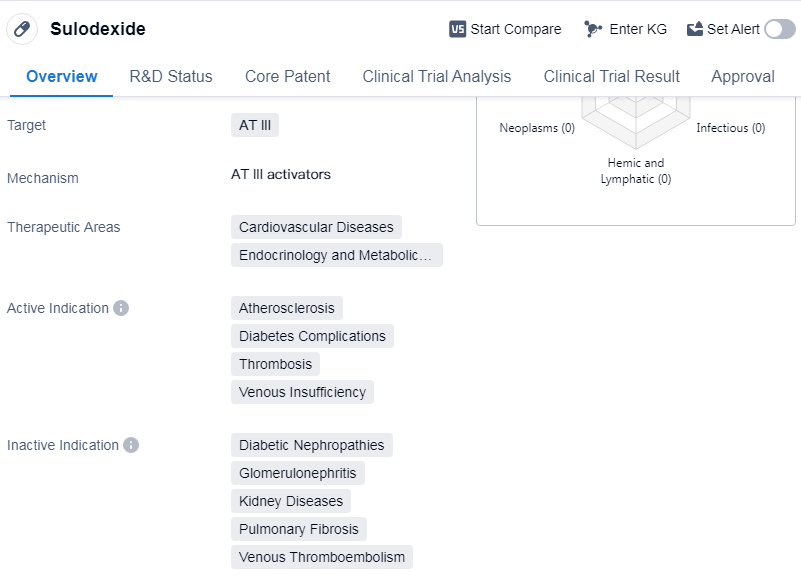
Sulodexide is a small molecule drug that targets AT III and is primarily used in the treatment of cardiovascular diseases, endocrinology, and metabolic diseases. It has been approved for use in various indications including atherosclerosis, diabetes complications, thrombosis, and venous insufficiency. The drug was first approved in China in August 2003 and has since received global approval.
Sulodexide is developed by Alfasigma SpA, an originator organization in the pharmaceutical industry. As a small molecule drug, it is designed to interact with the targetAT III in the body. This mechanism of action allows it to effectively address the cardiovascular diseases, endocrinology, and metabolic diseases.
The drug's active indications highlight its potential in treating atherosclerosis, a condition characterized by the buildup of plaque in the arteries, which can lead to heart attacks and strokes. Sulodexide also shows promise in managing diabetes complications, such as diabetic nephropathy and retinopathy, which can cause kidney and eye damage, respectively. Additionally, it is used in the treatment of thrombosis, a condition where blood clots form in the blood vessels, and venous insufficiency, a condition that affects the flow of blood from the legs back to the heart.
Sulodexide 's approval in China in 2003 suggests that it has been available to patients in China for a significant period of time, providing ample opportunity for real-world data collection and further evaluation of its benefits and potential side effects. With its approval globally, Sulodexide has demonstrated its safety and efficacy in clinical trials and regulatory assessments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sulodexide: AT III activators
AT III activators refer to substances or drugs that activate Antithrombin III (AT III), which is a naturally occurring protein in the body. Antithrombin III plays a crucial role in regulating blood clotting by inhibiting certain clotting factors. It helps prevent the formation of excessive blood clots, which can lead to conditions like deep vein thrombosis, pulmonary embolism, or stroke.
From a biomedical perspective, AT III activators are substances or drugs that enhance the activity of Antithrombin III. They can be used in medical interventions to treat or prevent thrombotic disorders, where there is an increased risk of abnormal blood clotting. By activating AT III, these activators promote the inhibition of clotting factors, thereby reducing the likelihood of clot formation.
AT III activators can be administered through various routes, including intravenous infusion or subcutaneous injection. They are commonly used in clinical settings, such as during surgery, in patients with hereditary or acquired AT III deficiency, or in individuals at high risk of thromboembolic events. These activators can help maintain the balance between clotting and anticoagulation, promoting proper blood flow and reducing the risk of complications associated with abnormal clotting.
Drug Target R&D Trends for Sulodexide
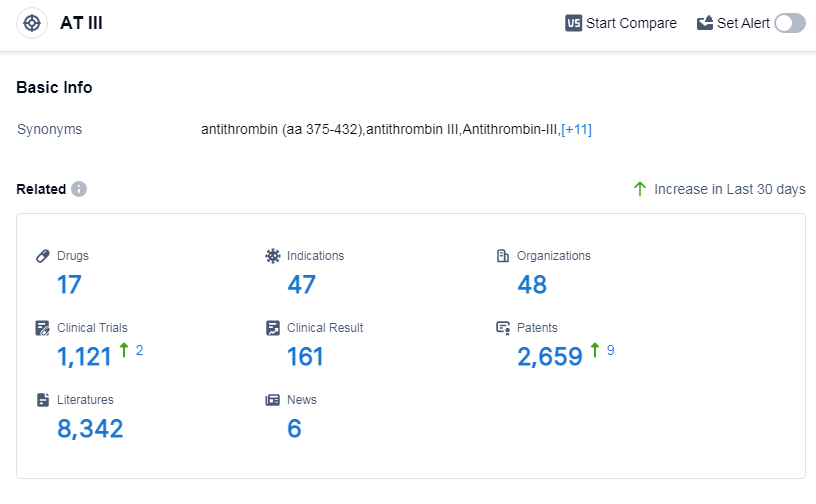
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 17 AT III drugs worldwide, from 48 organizations, covering 47 indications, and conducting 1121 clinical trials.
The analysis of target AT III reveals a competitive landscape with multiple companies actively involved in the research and development of drugs. Sanofi is leading in terms of the highest phase of development, with drugs in various stages. Indications such as venous thromboembolism, thrombosis, and pulmonary embolism have seen significant drug approvals. Chemical drugs are the most common type of drugs progressing under the target, with potential competition from biosimilars. China, the European Union, and Japan are the leading countries in terms of drug development for AT III. This analysis indicates a promising future for the target AT III, with ongoing research and development efforts to address various indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Sulodexide is a small molecule drug developed by Alfasigma SpA that targets AT III and is approved for use in the treatment of cardiovascular diseases, endocrinology, and metabolic diseases. Its active indications include atherosclerosis, diabetes complications, thrombosis, and venous insufficiency. The drug's approval in China in 2003 and subsequent global approval highlight its potential as a therapeutic option for patients in need.