GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (4)
This article will continue to introduce the GPCR (G Protein-Coupled Receptor) targets and pipelines that garnered attention for Chugai, UCB, Ascendis and BridgeBio at the JP Morgan Healthcare Conference 2024.For more details, please follow the link below.
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (1)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (2)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (3)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (5)
Chugai in JPM 2024
Chugai Pharmaceutical is a leading Japanese biopharmaceutical company, established in 1925 with its headquarters in Tokyo. As a member of the Roche Group, Chugai strives to bring Roche's extensive pipeline to the Japanese market, while simultaneously taking Chugai's rich portfolio of innovative drug research and development achievements to the global stage.
Chugai is dedicated to the research, development, and commercialization of innovative pharmaceuticals, achieving significant advancements particularly in the fields of oncology, immunology, orthopedics, and neuroscience. The company boasts robust internal R&D capabilities and actively collaborates with other global leading research institutions to undertake comprehensive drug development activities, from early-stage discovery to late-phase clinical trials.
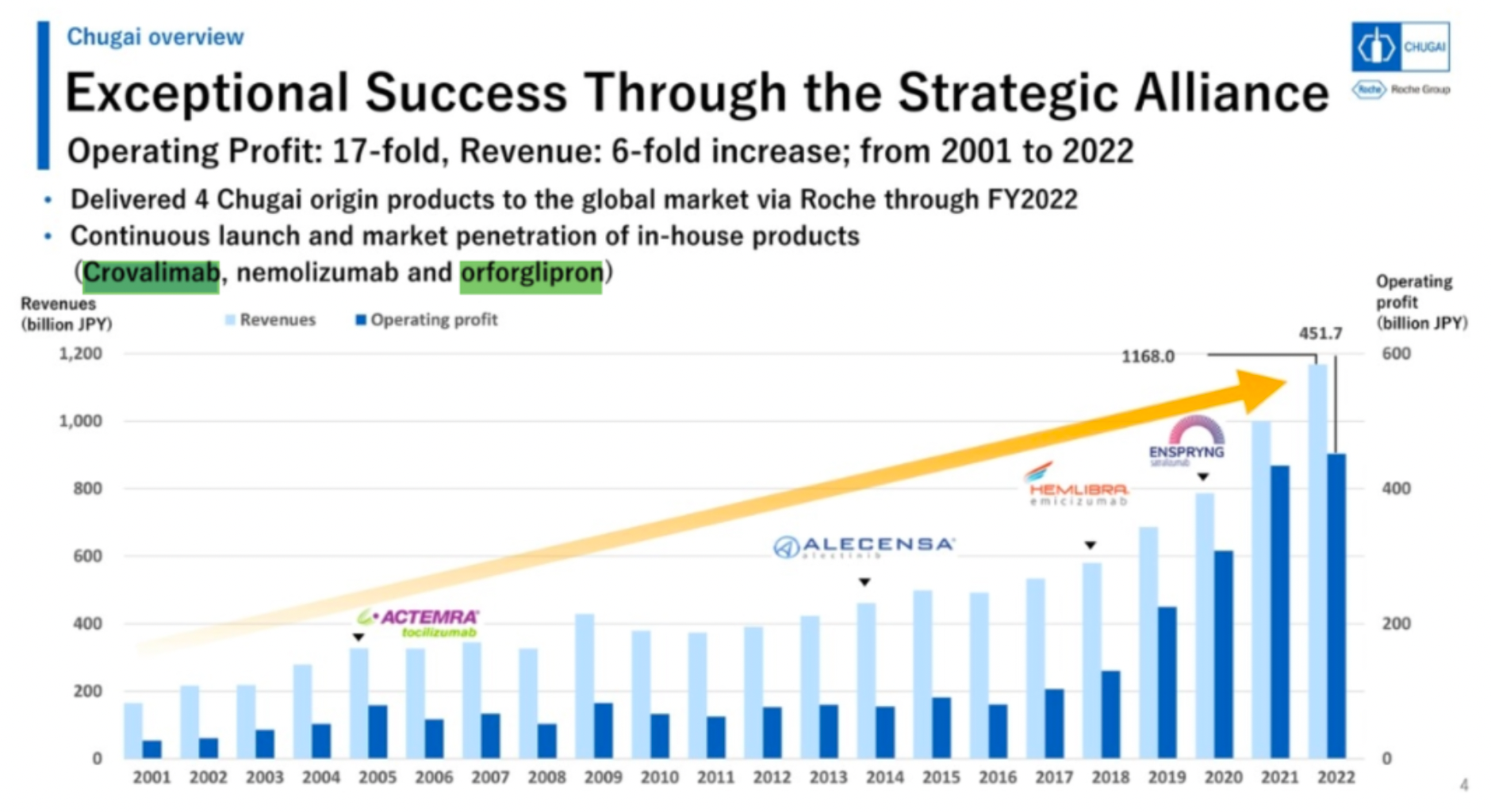
Through the formation of strategic alliances, Chugai has realized extraordinary success. From 2001 to 2022, the company's operating profit increased 17-fold, with revenue growing 6-fold. Chugai delivers four joint Sino-foreign products to the global market through Roche and continues to launch and infiltrate markets with proprietary products (Crovalimab, nemolizumab, and orforglipron).
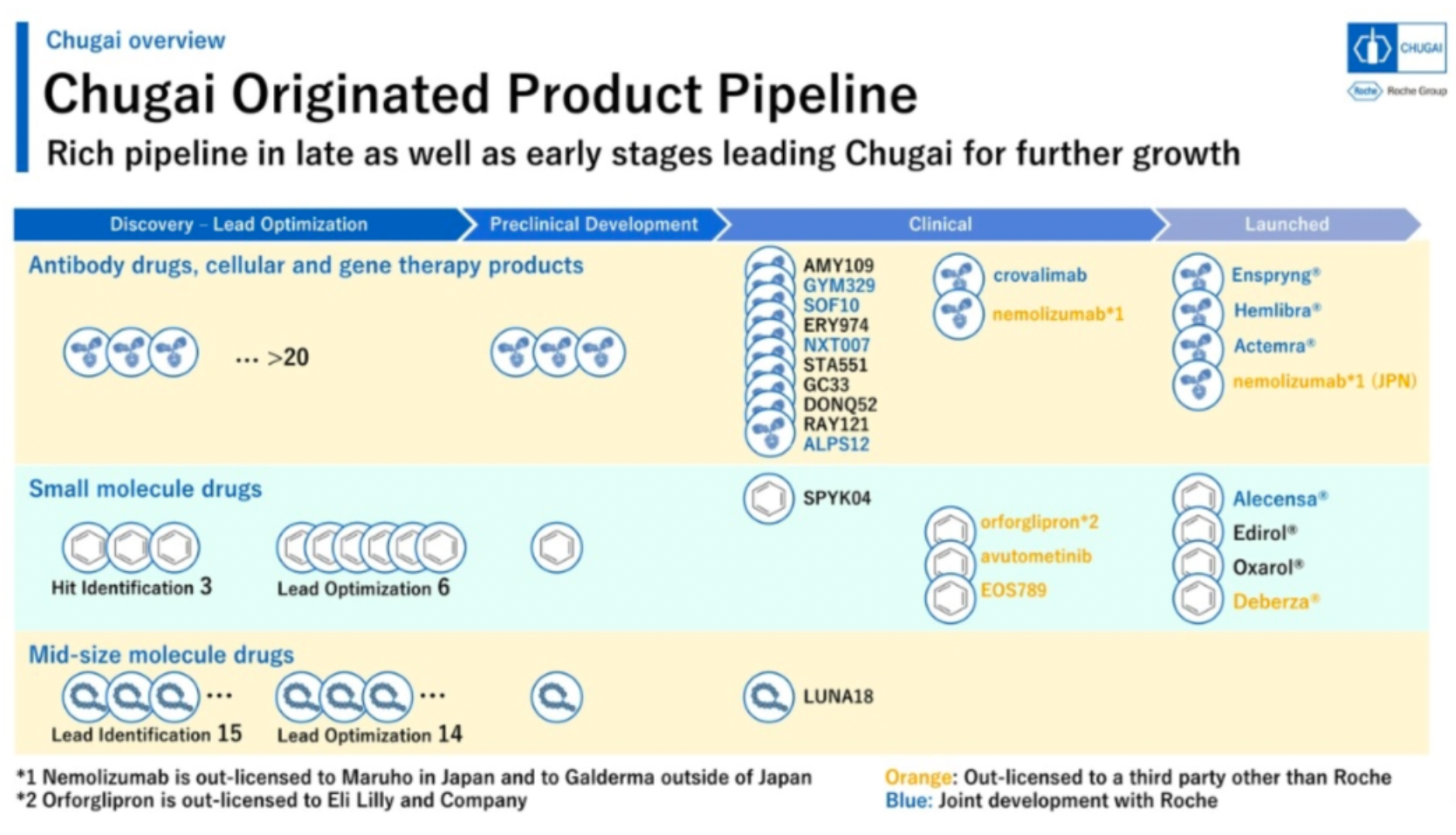
Chugai boasts an extensive pipeline of original research, with over 20 antibody drugs, cell therapy and gene therapy products in the drug discovery and preclinical research stages, 9 small molecule drugs, 29 mid-sized molecule drugs, 13 drugs in clinical stages, and 8 marketed drugs.
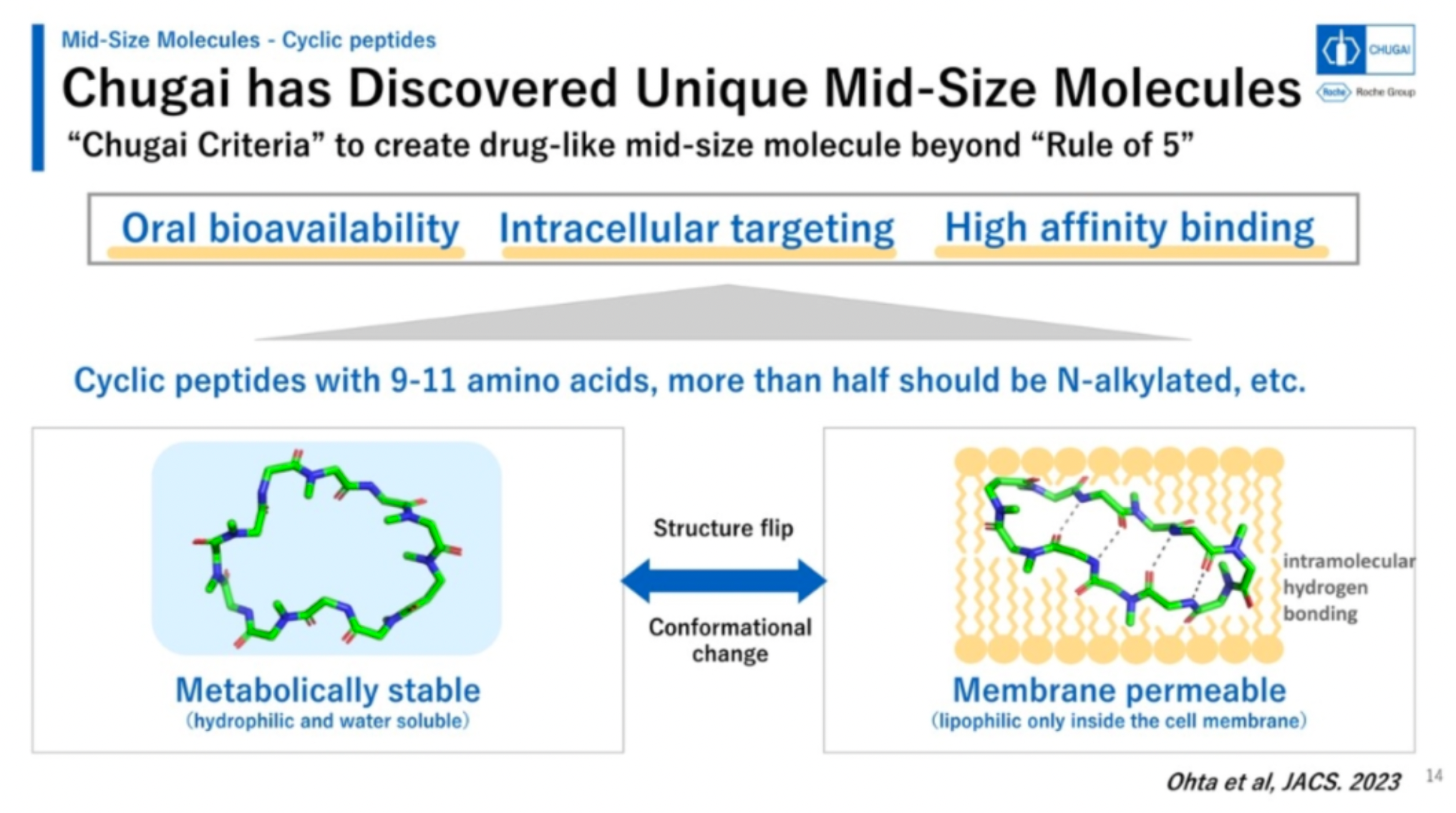
Chugai possesses robust R&D capabilities, and guided by the twin principles of technology-driven and quality-centered approaches, the company has established a range of unique proprietary drug molecules in various categories, including antibodies, small molecules, and cyclic peptide drugs.

In the field of antibodies, Chugai possesses 14 patented technologies.

Chugai's mid-sized cyclic peptide molecules exhibit a range of advantageous characteristics, including oral bioavailability, the ability to target intracellular targets, and high-affinity binding.
Orforglipron (OWL833, LY3502970) is a small molecule GLP-1R agonist developed by Chugai Pharmaceuticals. In 2018, Eli Lilly and Company signed a collaboration agreement with Chugai, paying a $50 million upfront payment to acquire the rights for the clinical development of Orforglipron for the treatment of type 2 diabetes. Between August and September of 2023, Eli Lilly published four clinical research trial data articles sequentially in "The Lancet," "The New England Journal of Medicine," and "Diabetes, Obesity and Metabolism," presenting the results of phase 1a, 1b, and 2 trials of Orforglipron for the treatment of type 2 diabetes and obesity.
UCB in JPM 2024
UCB Inc. is a global biopharmaceutical enterprise dedicated to developing innovative drugs for the treatment of serious diseases in the fields of immunology and neuroscience. The company's goal is to become the 'patient-preferred' leader in the biopharmaceutical sector by creating patient value for specific communities through unique outcomes and the ultimate experience, and to improve their lives as much as possible.
In 2023, the company's blockbuster product CIMZIA achieved its sales peak target of 2 billion euros ahead of schedule, and with a differentiated advantage, its sales growth outperformed the anti-TNF market. It is anticipated that there will be no competition from biosimilars until before 2027.
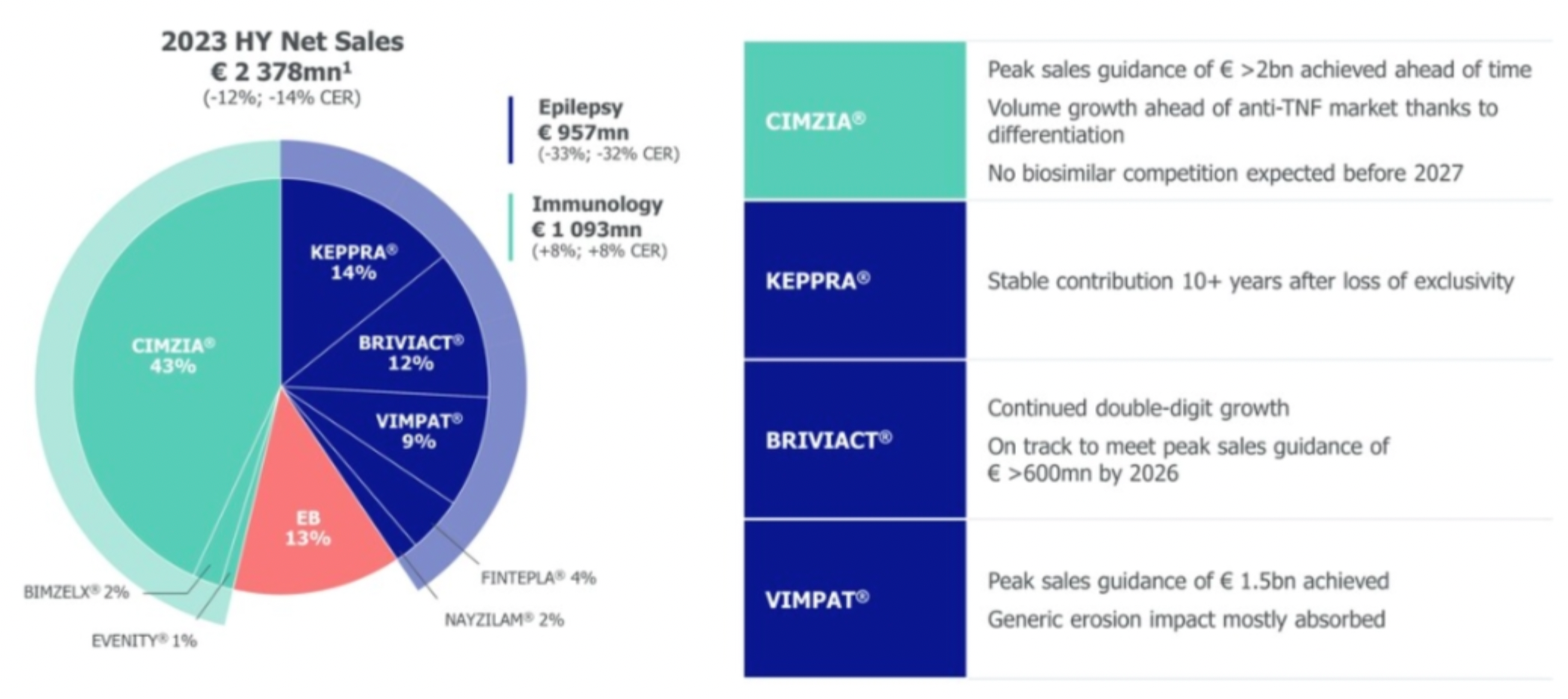
Additionally, five products, including EVENITY®, BIMZELX®, FINTEPLA®, RYSTIGGO®, and ZILBRYSQ®, may possess sales growth momentum for up to ten years.

UCB has announced several R&D pipeline candidates that are expected to reach significant clinical data milestones in 2024.
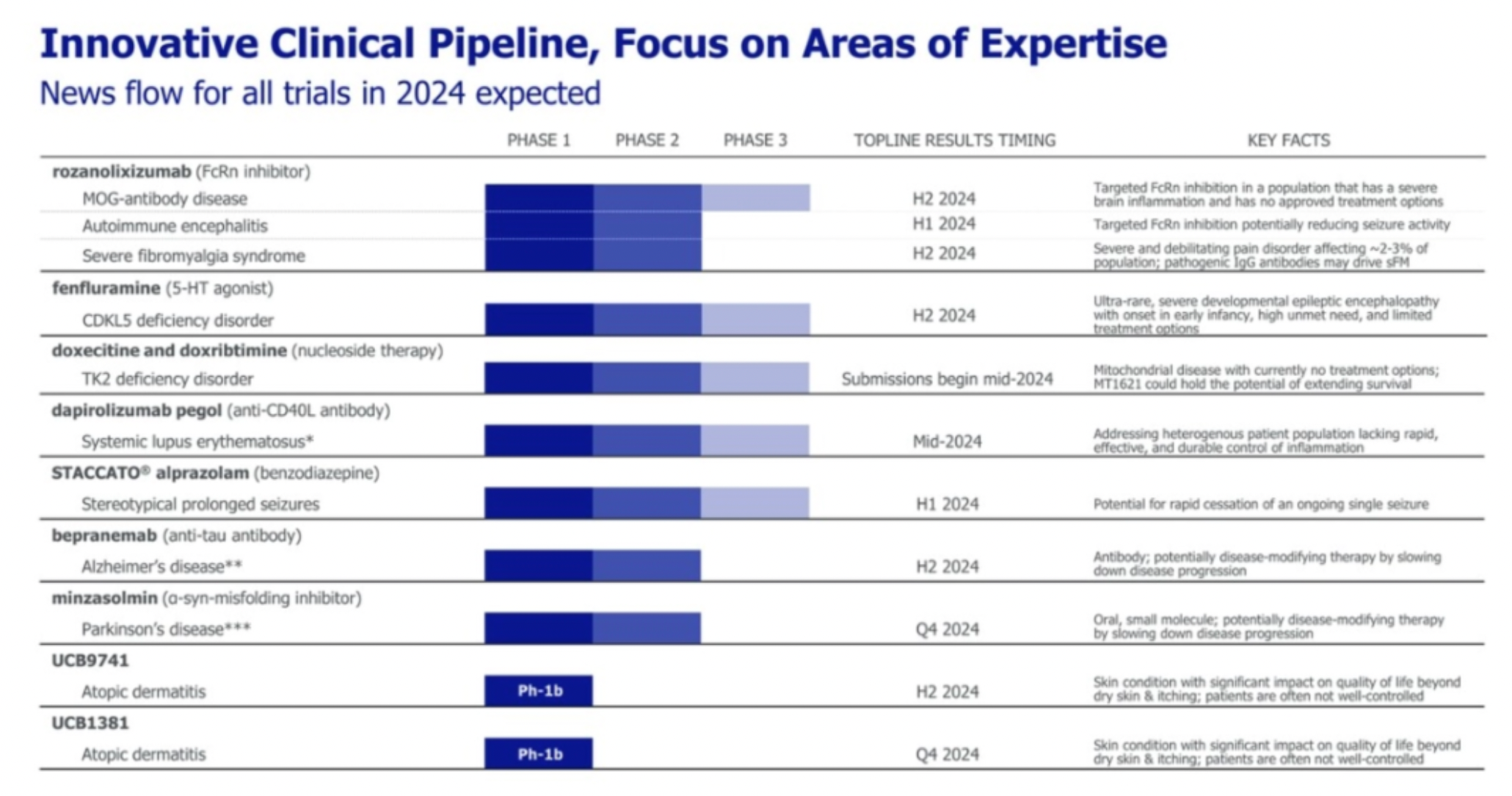
Fenfluramine Hydrochloride is a multi-target small molecule drug developed by Zogenix. Its mechanism of action functions as an agonist for 5-HT1D receptors, 5-HT2A receptors, and 5-HT2C receptors, among others. The medication was approved for marketing in 2020 for the treatment of Dravet syndrome and epilepsy. In 2022, UCB announced the acquisition of Zogenix at an approximate total cost of 1.9 billion US dollars.
Ascendis in JPM 2024
Ascendis Pharma is a global biopharmaceutical company focused on developing innovative and differentiated therapies for rare diseases, using its proprietary TransCon (Transient Conjugation) technology platform to design and manufacture drugs. This platform creates compounds that are temporarily bound (or "transiently conjugated"), allowing for precise control over the drug's release behavior in the body.
The TransCon technology enables the activation of the drug after it enters the body, with the release of the active therapeutic ingredient at predetermined times and locations. This technology offers new treatment options for various diseases, especially for patients with rare diseases who require precise drug exposure to achieve optimal therapeutic effects and minimize the risk of side effects.
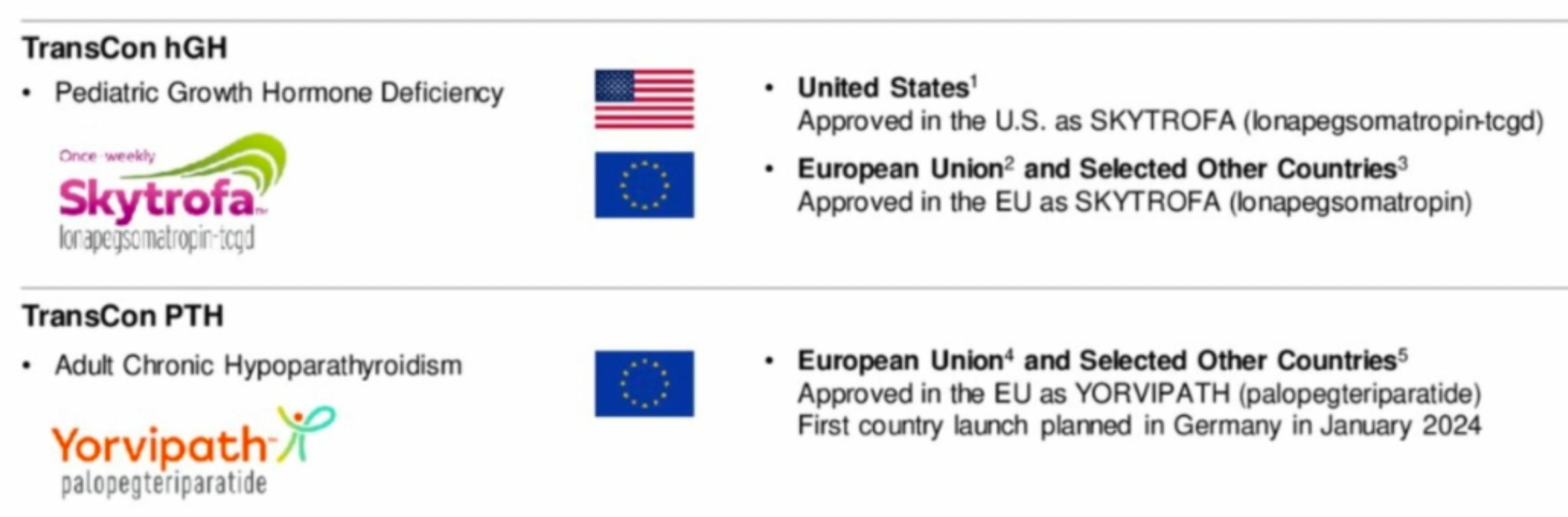
The company's leading endocrinology rare disease products, including TransCon hGH and TransCon PTH, have been approved in major markets.
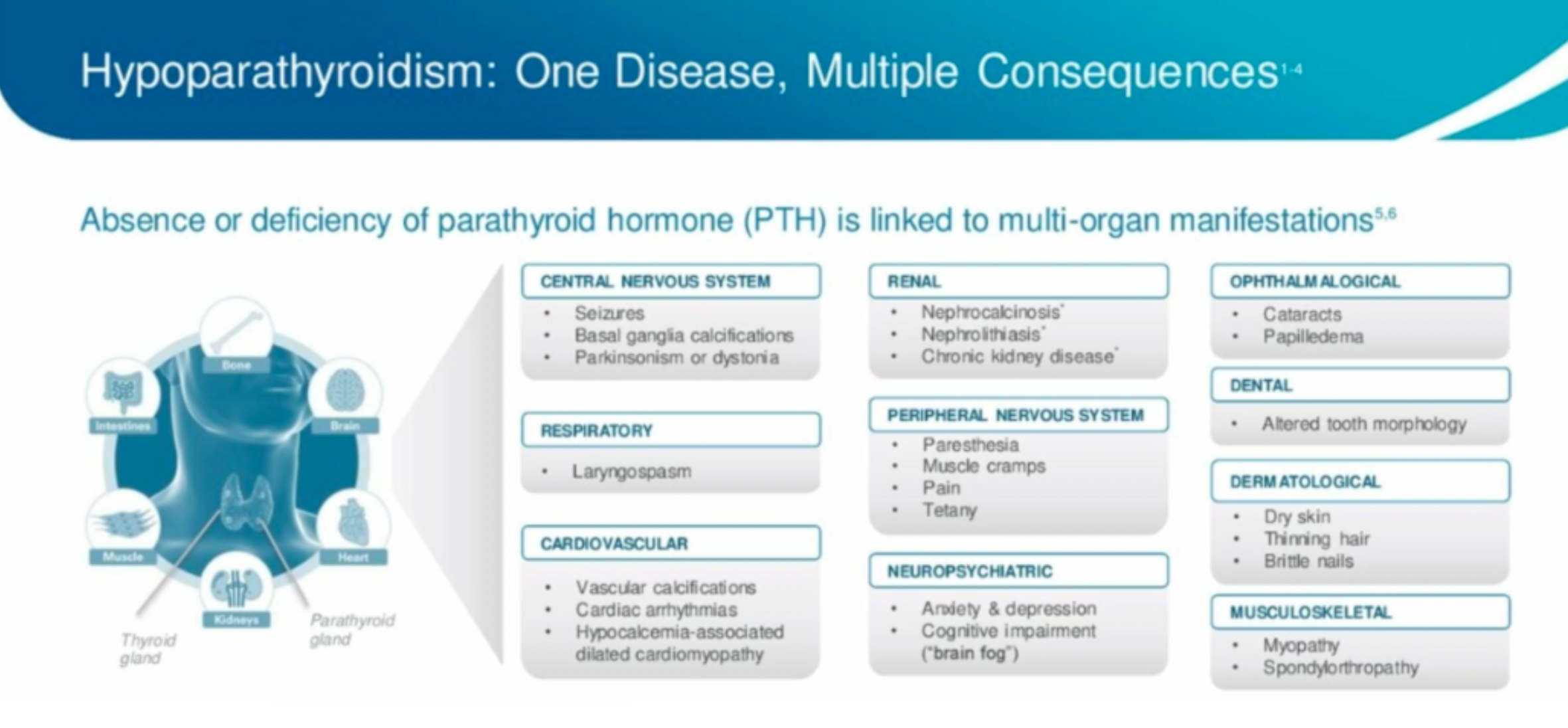
Hypoparathyroidism is an endocrine disorder caused by insufficient or absent secretion of parathyroid hormone (PTH), which can affect multiple organs. Under normal physiological conditions, PTH and its downstream hormone calcitriol are the main regulators of calcium and phosphate, acting on bones, kidneys, and intestines. Hypoparathyroidism can lead to hypocalcemia and hyperphosphatemia.
TransCon PTH is an investigational prodrug of PTH hormone originally developed by Ascendis Pharma that is administered via a once-daily injection to provide sustained release of active PTH. It is currently under development for the treatment of adult hypoparathyroidism. This medication consists of the parent drug PTH(1-34) transiently linked to a branched methoxy polyethylene glycol (mPEG) carrier using a proprietary linker. The drug is administered through a once-daily subcutaneous injection, ensuring stable PTH levels within a normal physiological range over 24 hours. By activating downstream PTH1R receptors, it modulates function.
BridgeBio in JPM 2024
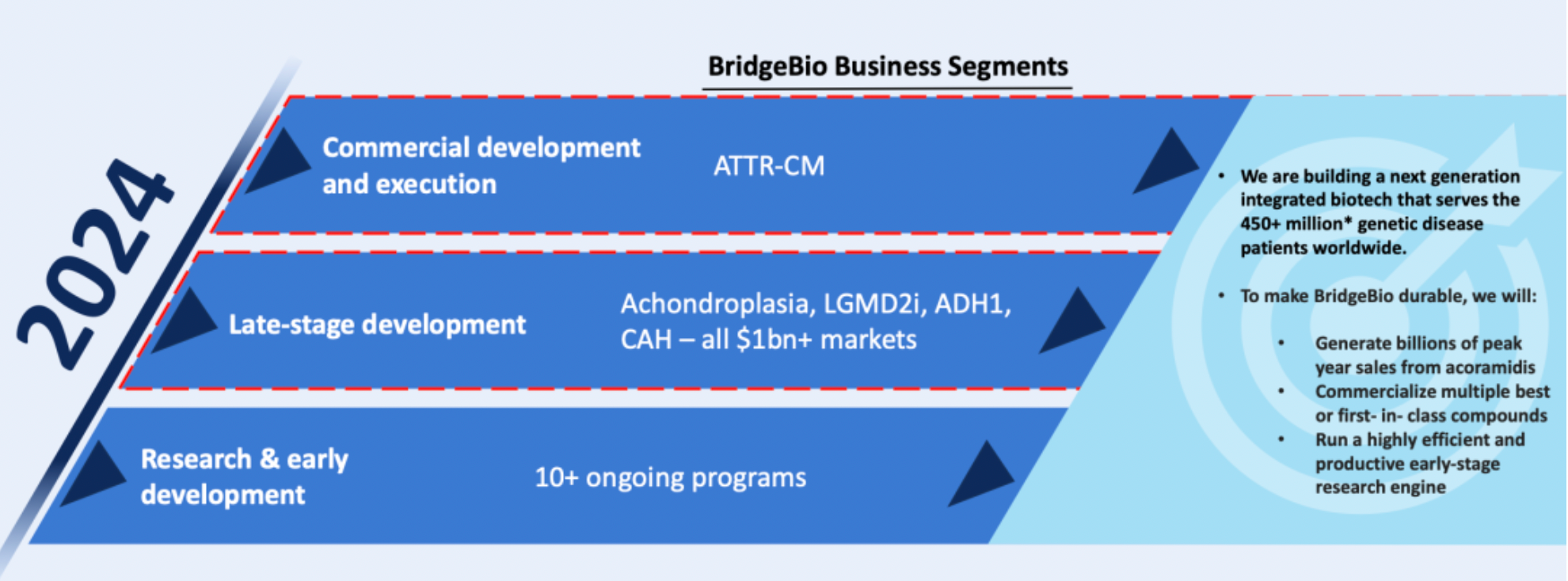
BridgeBio is a next-generation biotechnology company specializing in the treatment of genetic diseases, dedicated to providing innovative therapies to over 450 million patients worldwide suffering from genetic disorders. The company's operations encompass every stage from early research and development to commercialization, with a deep focus on drug development for rare disease areas that have market potential exceeding one billion US dollars.
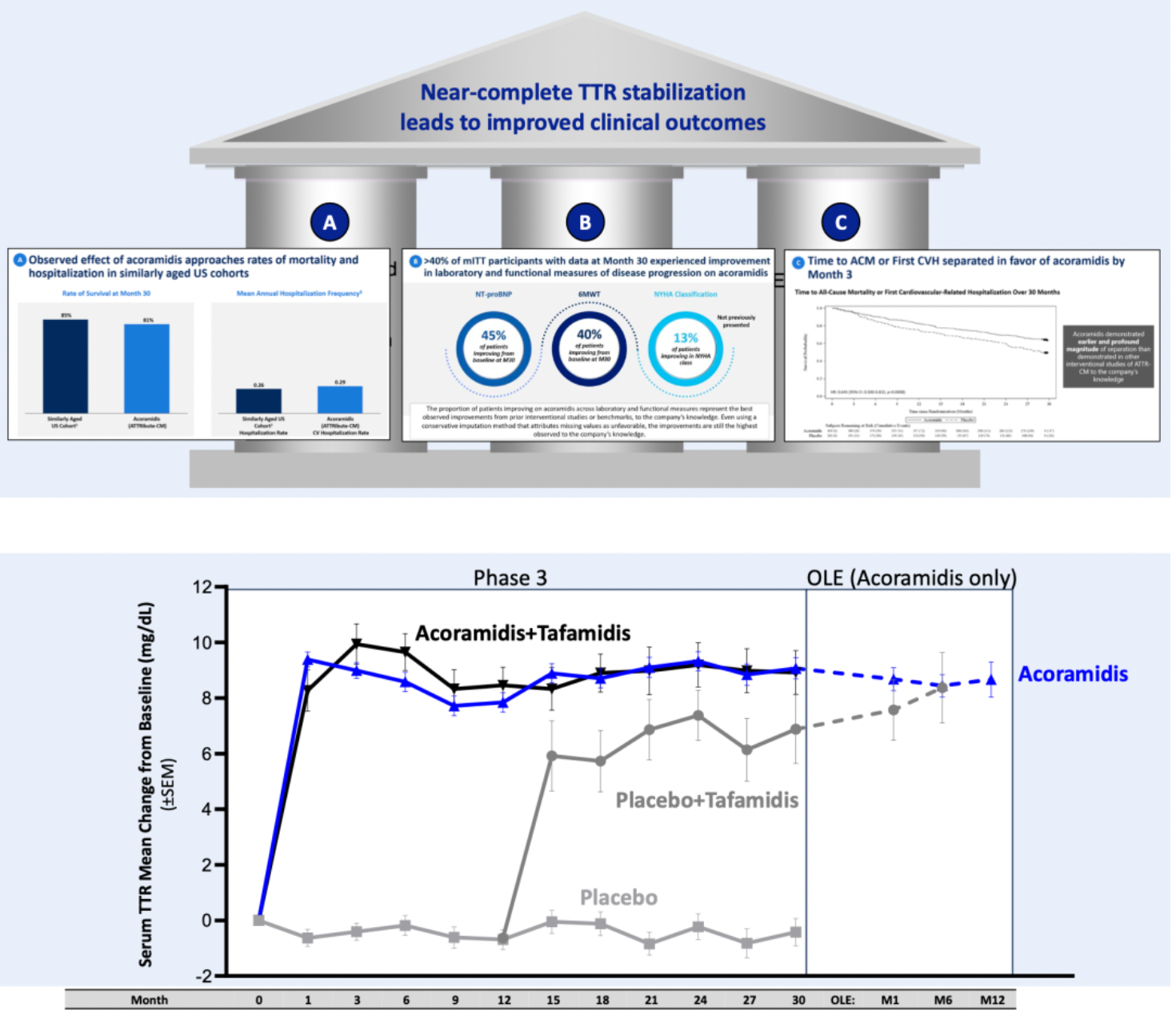
One of BridgeBio's key products currently in development is acoramidis, intended for the treatment of ATTR-CM (transthyretin amyloidosis cardiomyopathy).
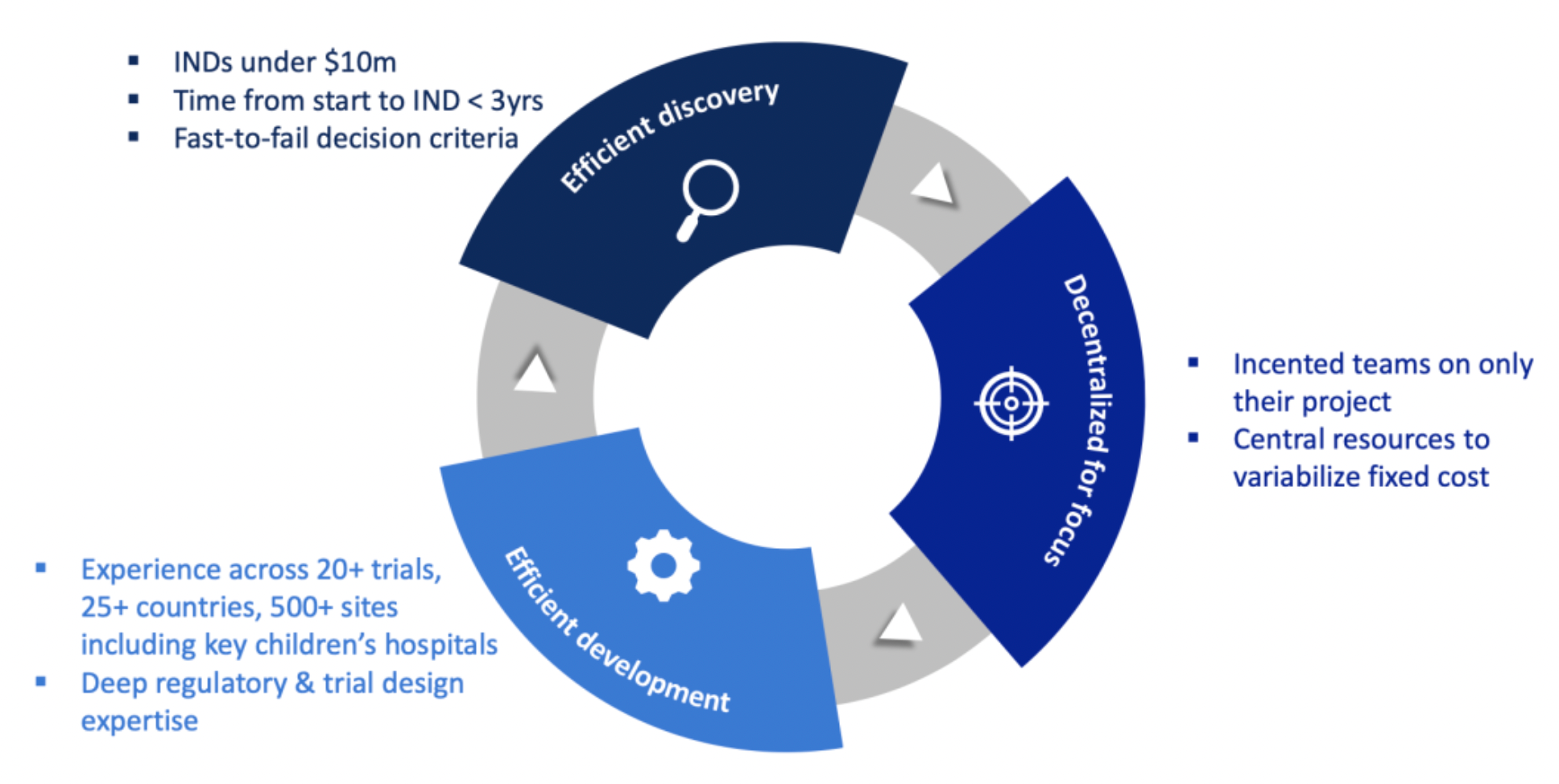
BridgeBio’s R&D engine is specifically designed for genetic medicines, featuring efficiency, low cost, and rapid decision-making capabilities that allow the advancement of new drug clinical trial applications (IND) at a cost of less than 10 million US dollars, and achieving IND filing in under three years. The company has significant experience in clinical trials, operating in more than 20 trials globally, across 25 countries and over 500 sites, with a particular focus on collaborations with key pediatric hospitals.
According to the Phase 3 clinical trial data released by BridgeBio, patients treated with acoramidis demonstrated an unprecedented improvement in absolute survival rates, meaning that patients lived longer and had a significant reduction in hospitalization needs due to the disease, approaching the level seen in healthy individuals of the same age. Additionally, acoramidis showed early divergence from the control group in cardiovascular-related mortality and heart failure composite outcomes within just three months, which is the earliest known time point for observing treatment effects to date.
The company's clinical candidate drug Encaleret is indicated for the treatment of Autosomal Dominant Hypocalcemia type 1 (ADH1). This is a rare genetic disorder caused by gain-of-function mutations in the calcium-sensing receptor (CaSR) gene, leading to dysregulated secretion of parathyroid hormone (PTH) and resulting in hypocalcemia.
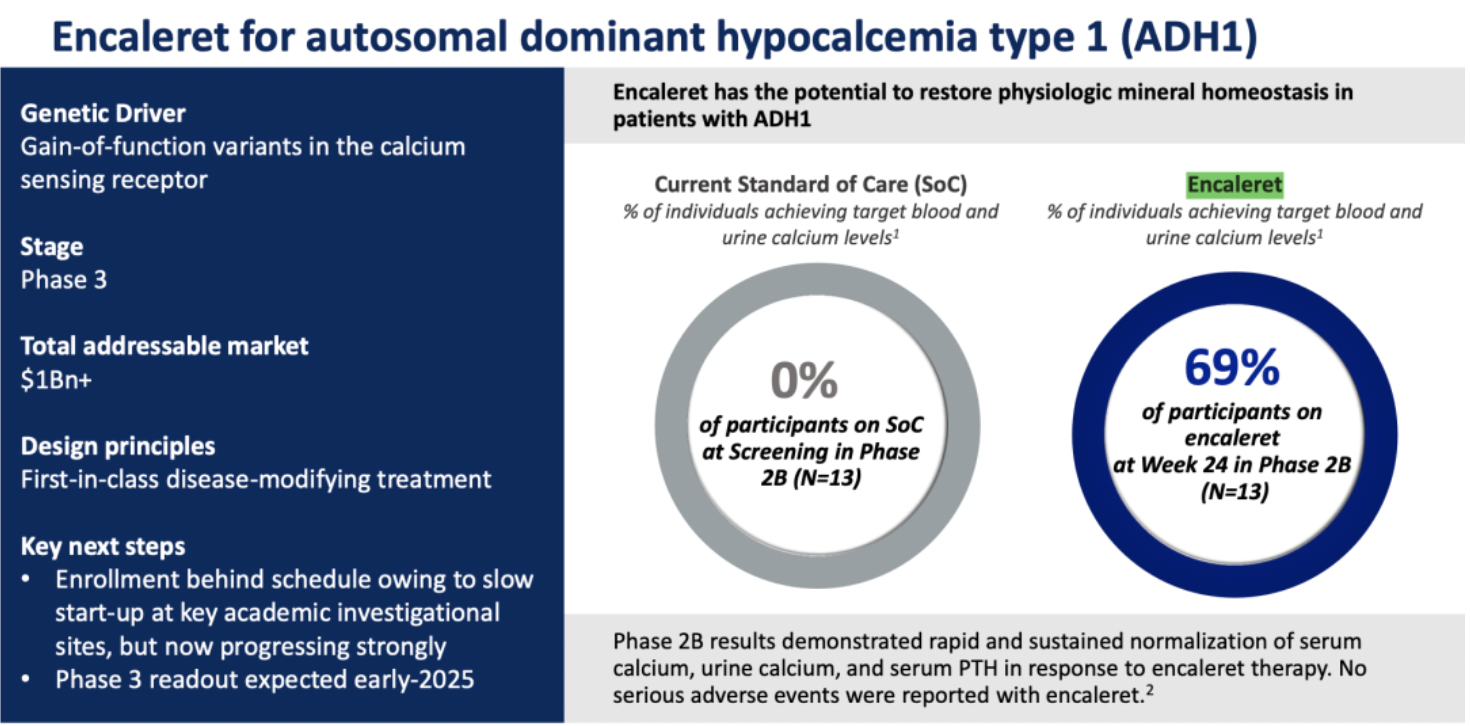
Encaleret sulfate is a product that acts as an antagonist targeting the CaSR receptor. As a potential "first-in-class" disease-modifying therapeutic agent, it is designed to restore physiological mineral homeostasis, assisting ADH1 patients in achieving normal levels of serum calcium and urinary calcium.