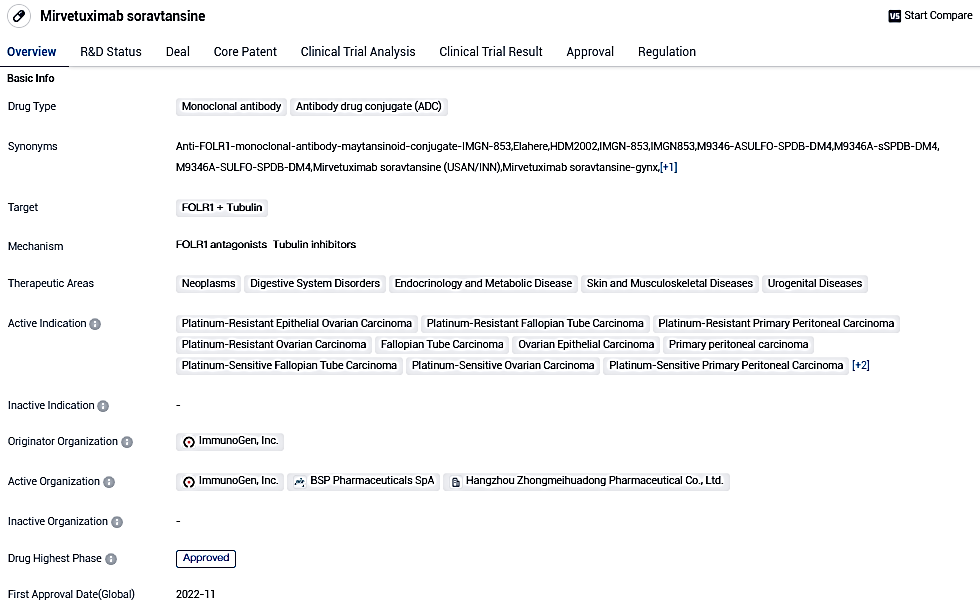
ImmunoGen Announces EMA Approval of Mirvetuximab Soravtansine's Application for Treating Platinum-Resistant Ovarian Cancer
ImmunoGen, Inc., prominent in the growing sector of antibody-drug conjuncts for cancer therapy, declared today that EMA has acknowledged the Marketing Authorization Application for mirvetuximab soravtansine (ELAHERE). This treatment is intended for patients suffering from folate receptor alpha (FRα)-positive, platinum-resistant epithelial ovarian, fallopian tube, or initial peritoneal cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Our MAA approval marks a significant step forward in the future progression of ELAHERE. We are committed to ensuring this potentially transformative treatment reaches patients battling platinum-resistant ovarian cancer worldwide," stated Michael Vasconcelles, MD, ImmunoGen's Executive Vice President, Research, Development, and Medical Affairs.
"Taking pride in being the pioneering medicine to show a higher survival rate in platinum-resistant ovarian cancer against chemotherapy in a Phase 3 clinical study, we are delighted to kickstart the review process that gets us nearer to providing eligible patients in Europe with access to ELAHERE. We anticipate a fruitful collaboration with the EMA during the review process and hope to introduce this innovative ADC to Europe possibly by the year 2024," Michael Vasconcelles added.
The positive results from the Phase 3 MIRASOL trial, revealed in May 2023, study of ELAHERE’s effectiveness against platinum-resistant ovarian cancer backup the MAA. This data was later on delivered as a late-breaking abstract at the annual 2023 American Society of Clinical Oncology Meeting.
The MIRASOL trial showed meaningful and statistically significant enhancements in progression-free survival, objective response rate, and overall survival by ELAHERE when compared to single-agent chemotherapy selected by investigators. ELAHERE displayed a manageable safety profile compared to IC chemotherapy, with occurrences of primarily mild eye and gastrointestinal symptoms.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
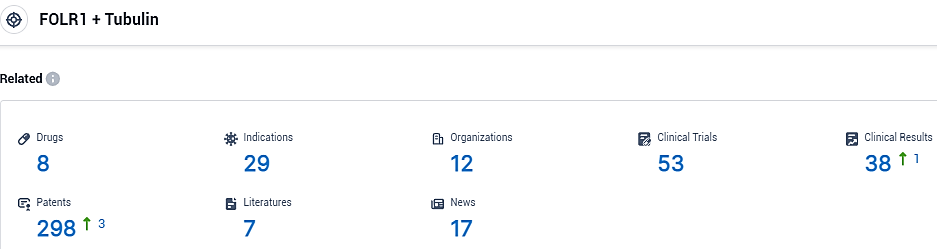
According to the data provided by the Synapse Database, As of October 31, 2023, there are 8 investigational drugs for the FOLR1 and tubulin target, including 29 indications, 12 R&D institutions involved, with related clinical trials reaching 53, and as many as 298 patents.
ELAHERE has been designated for administering to adults suffering from FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have undergone one to three earlier systemic therapeutic procedures. The selection of patients for the treatment should follow an FDA-approved test. The approval of this indication leans on an accelerated basis considering the tumor response rate and duration of this response. The sustained approval of this indication might depend on the subsequent verification and elucidation of the clinical advantage in a subsequent confirmatory trial.