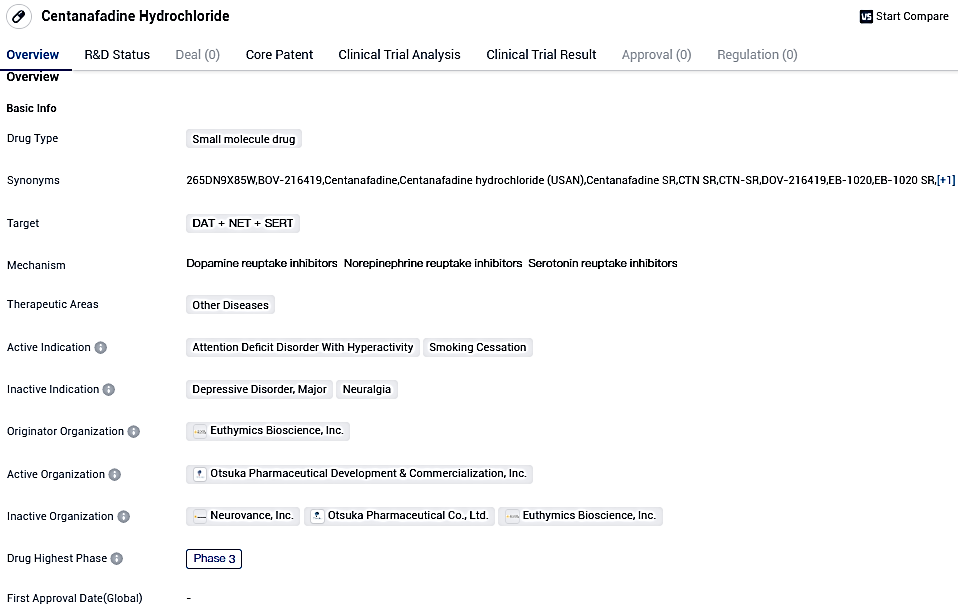
Otsuka Pharmaceutical unveils Phase 3 studies of Centanafadine for treating Attention Deficit Hyperactivity Disorder in children and teenagers
Otsuka Pharmaceutical Co., Ltd. along with its American branch, Otsuka Pharmaceutical Development & Commercialization, Inc., has declared encouraging findings from two Phase 3 clinical investigations. These six-week trials assessed the effectiveness, safety, and tolerance of centanafadine in managing attention-deficit/hyperactivity disorder in children and teens. Centanafadine, a pioneer in the category of norepinephrine, dopamine, and serotonin reuptake inhibitors, was used in these trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
There were similarities in the structure of the trials; each one was a three-pronged, double-blind, fixed-dose investigation where patients were allocated randomly to either receive a low dosage of centanafadine, a high dosage of centanafadine, or a placebo.
The primary trial was an instrumental Phase 3, randomly allocated, covered-eye, trifurcated, fixed-dose test to determine the effectiveness, safety, and palatability of centanafadine for teenagers with ADHD, ranging from 13 to 17 years old.
The secondary trial was an instrumental Phase 3, randomly allocated, covered-eye, trifurcated, fixed-dose test to determine the effectiveness, safety, and palatability of centanafadine for younger subjects with ADHD, aged 6 to 12 years old.
“Otsuka stays committed to discovering innovative methods for intricate medical problems that are underrepresented,” John Kraus, M.D., Ph.D., the chief medical officer and executive vice president of Otsuka Pharmaceutical Development & Commercialization, Inc., speaks out. “Our satisfaction comes from the instrumental Phase 3 results, suggesting that centanafadine might herald a new remedy for children and adolescents living with ADHD, a condition that can permeate all aspects of life.”
“Otsuka continues to commit to the discovery of innovative approaches to complicated and inadequately served medical needs,” John Kraus, M.D., Ph.D., executive vice president and chief medical officer at Otsuka Pharmaceutical Development & Commercialization, Inc., states. “The Phase 3 crucial data suggests that centanafadine could potentially provide a novel medical treatment for children and teenagers suffering from ADHD, a condition that has life-long ramifications.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
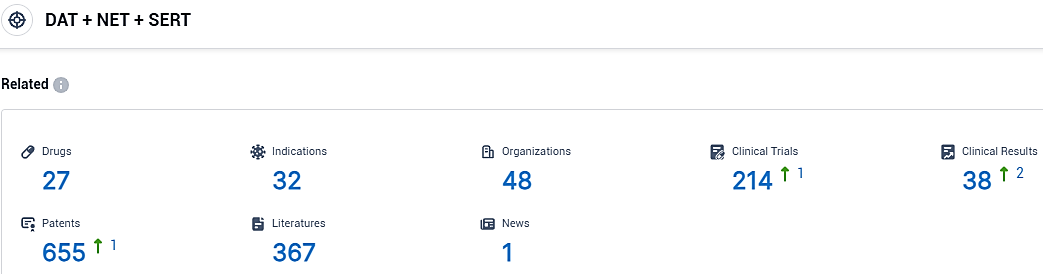
According to the data provided by the Synapse Database, As of October 31, 2023, there are 27 investigational drugs for the DAT and NET and SERT target, including 32 indications, 48 R&D institutions involved, with related clinical trials reaching 214, and as many as 655 patents.
Centanafadine focuses on DAT/NET/SERT and is under examination for its prospective application in the management of ADHD and help in quitting smoking. Currently in the third phase, the medication has demonstrated potential in previous development stages and could also be beneficial in the cure of other illnesses.