An In-depth Analysis of Midodrine Hydrochloride's R&D Progress and Mechanism of Action on Drug Target
Midodrine Hydrochloride's R&D Progress
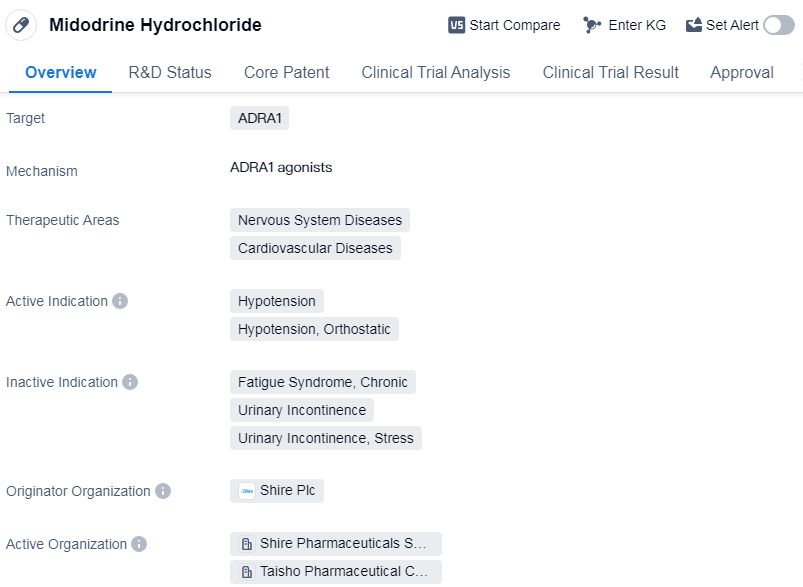
Midodrine Hydrochloride is a drug that targets the ADRA1 receptor and is used in the treatment of nervous system diseases and cardiovascular diseases. Its active indications include hypotension, particularly orthostatic hypotension. The drug was developed by Shire Plc and has been approved for use in multiple countries, including Switzerland.
Midodrine Hydrochloride was first approved for use in 1987 in Switzerland. It is classified as an orphan drug, which means it is used to treat rare diseases or conditions that affect a small number of people. The drug has reached the highest phase of approval globally.
The ADRA1 receptor is a target for Midodrine Hydrochloride, and it is believed to work by stimulating this receptor, leading to vasoconstriction and an increase in blood pressure. This mechanism of action makes it effective in treating hypotension, a condition characterized by low blood pressure. Orthostatic hypotension, which is a drop in blood pressure upon standing up, is also treated with this drug.
Nervous system diseases and cardiovascular diseases are the therapeutic areas in which Midodrine Hydrochloride is used. These conditions can have a significant impact on a person's health and quality of life, and the drug provides a treatment option for patients suffering from these diseases.
As an orphan drug, it is likely that the development and approval process for this drug involved specific incentives and regulations to encourage its development for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Midodrine Hydrochloride: ADRA1 agonists
ADRA1 agonists are a type of drug that act on the alpha-1 adrenergic receptors in the body. These receptors are found in various tissues and organs, including the smooth muscles of blood vessels. When ADRA1 agonists bind to these receptors, they stimulate them and elicit a response.
From a biomedical perspective, ADRA1 agonists can have several effects on the body. By activating alpha-1 adrenergic receptors in blood vessels, these drugs can cause vasoconstriction, which leads to the narrowing of blood vessels. This can be beneficial in certain medical conditions, such as hypotension (low blood pressure), where vasoconstriction helps increase blood pressure.
ADRA1 agonists can also have effects on other tissues and organs. For example, in the urinary system, these drugs can stimulate alpha-1 adrenergic receptors in the smooth muscles of the bladder neck and prostate, leading to relaxation of these muscles. This effect can be useful in the treatment of conditions like benign prostatic hyperplasia (BPH) or urinary retention.
In summary, ADRA1 agonists are drugs that activate alpha-1 adrenergic receptors in the body, leading to various physiological responses. These drugs can be used in the management of conditions such as hypotension, BPH, or urinary retention.
Drug Target R&D Trends for Midodrine Hydrochloride
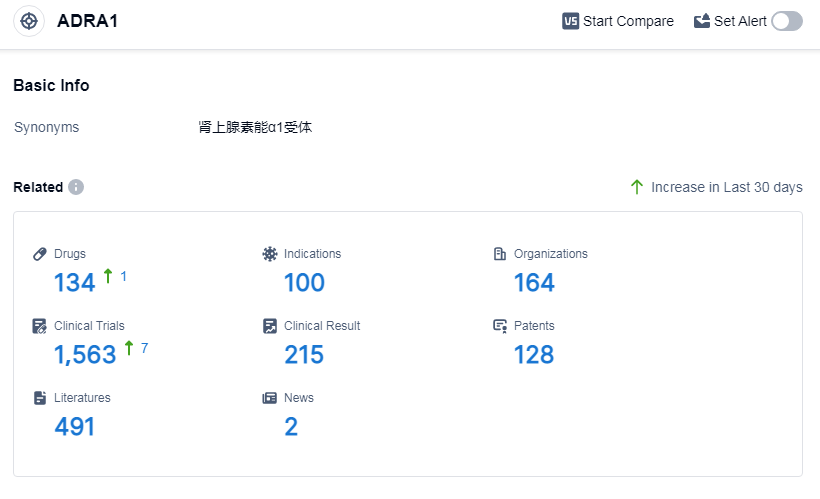
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 134 ADRA1 drugs worldwide, from 164 organizations, covering 100 indications, and conducting 1563 clinical trials.
In conclusion, the analysis of target ADRA1 reveals that GSK Plc, Pfizer Inc., Sanofi, Merck & Co., Inc., and Eisai Co., Ltd. are the companies growing fastest under this target. The indications with the highest number of approved drugs are hypertension, nasal obstruction, and common cold. Small molecule drugs are progressing most rapidly under the target ADRA1, indicating intense competition around innovative drugs. China, the United States, Japan, and the European Union are the countries/locations developing fastest under this target, with China showing significant progress. The current competitive landscape suggests a strong focus on developing drugs for hypertension, nasal obstruction, and common cold, primarily in the form of small molecule drugs. The future development of target ADRA1 is expected to be driven by the continuous efforts of these companies and countries/locations, with China playing a significant role in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Midodrine Hydrochloride is a drug that targets the ADRA1 receptor and is used in the treatment of nervous system diseases and cardiovascular diseases. It has been approved for use since 1987 and is classified as an orphan drug. Its active indications include hypotension and orthostatic hypotension. Developed by Shire Plc, Midodrine Hydrochloride provides a treatment option for patients suffering from these conditions.