Rona Therapeutics' RN0191 siRNA Treatment Shows Promise in Phase 1 at 2024 AHA
Rona Therapeutics, a pioneer in advanced RNA-focused treatments, announced favorable findings from the Phase 1 clinical trial of RN0191 during the American Heart Association's (AHA) Annual Scientific Sessions held in Chicago, Illinois, on November 16th, 2024. RN0191 is a unique GalNAc conjugated PCSK9 siRNA aimed at substantially reducing low-density lipoprotein cholesterol (LDL-C) and various lipid metrics.
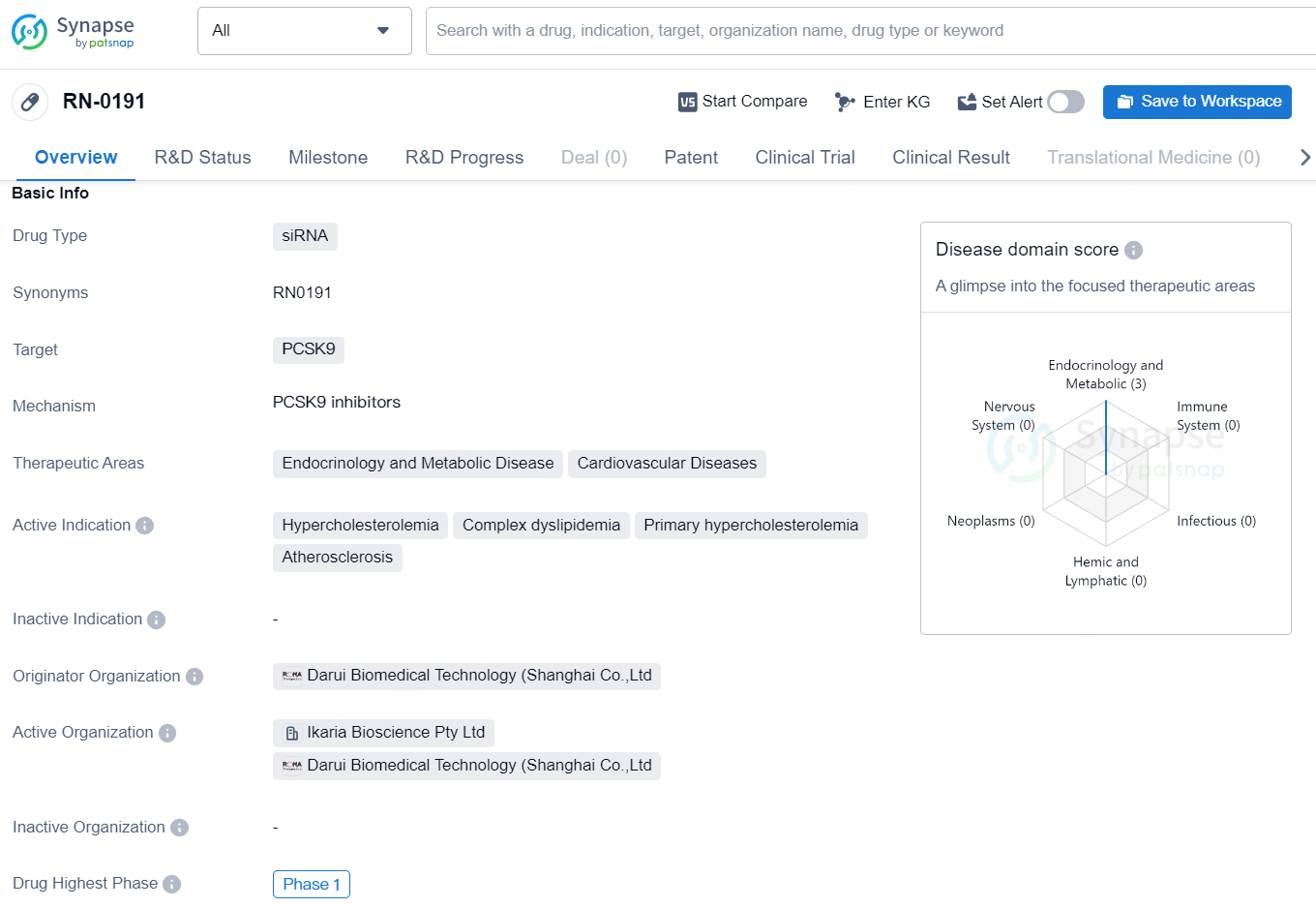
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 1 trial was a randomized, single-dose ascending, placebo-controlled investigation aimed at assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy individuals with elevated LDL-C levels. Participants consisted of adults aged between 18 and 60, with a BMI ranging from 19 to 30 kg/m2. All findings are derived from the database records as of October 14, 2024. A total of 32 participants were randomized and received RN0191 at doses ranging from 60 mg to 600 mg. The baseline average LDL-C levels were between 110 and 130 mg/dL.
Following a single subcutaneous injection, RN0191 demonstrated a favorable safety profile, with no severe adverse events reported and only mild, temporary adverse effects noted across all dosing levels. Notable dose-dependent and significant changes in PCSK9, LDL-C, and other lipid metrics were recorded (refer to the table below).
An average maximum reduction in PCSK9 exceeding 85% and LDL-C reductions of over 55% were achieved. A significant and sustained reduction in LDL-C of up to 42% was observed by Day 180, suggesting the potential for a bi-annual treatment schedule in future development. Additionally, substantial reductions in ApoB, Lp(a), non-HDL-C, and total cholesterol were also observed, with impressive treatment effects on PCSK9, LDL-C, and other lipid levels maintained for up to six months.
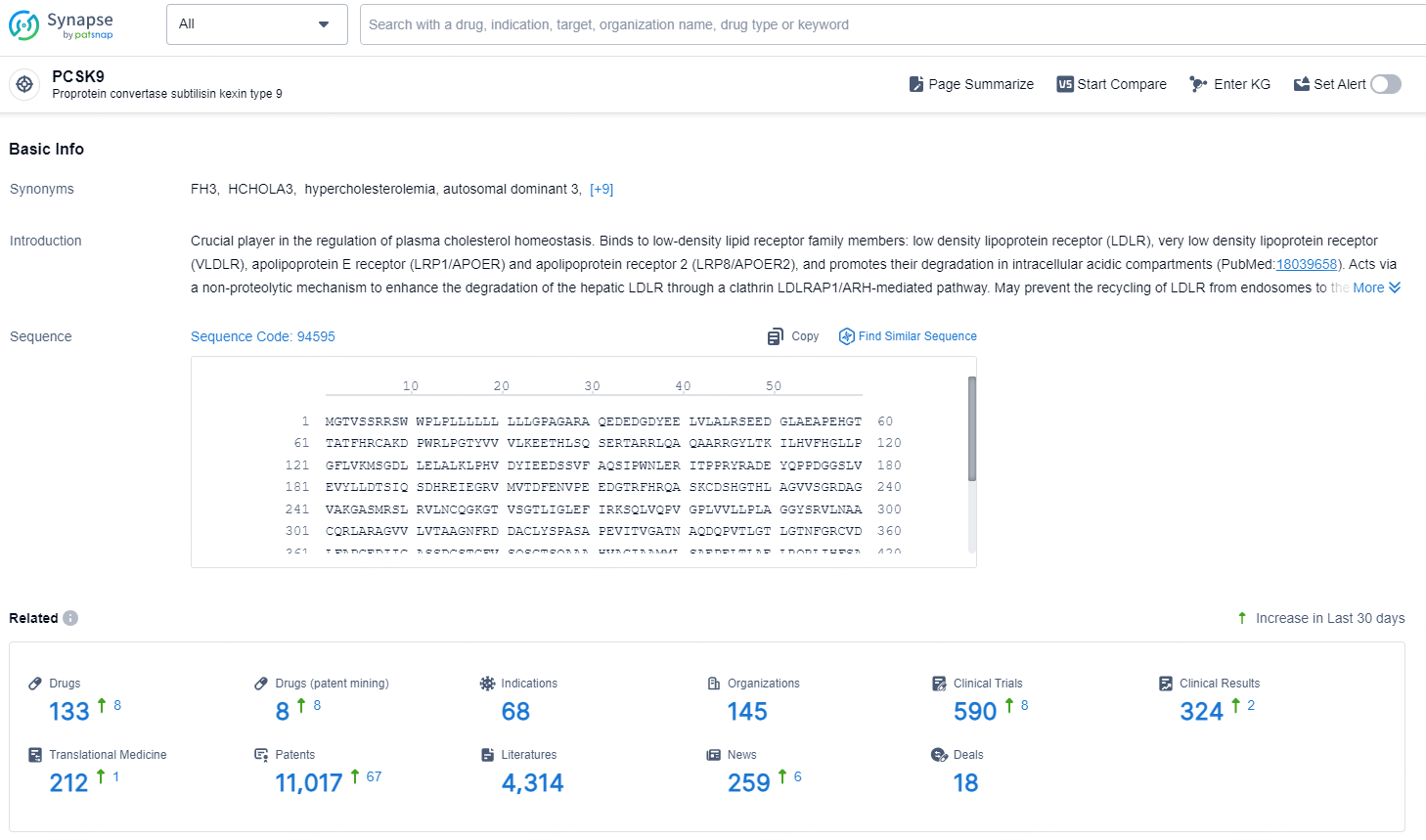
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 133 investigational drugs for the PCSK9 target, including 68 indications, 145 R&D institutions involved, with related clinical trials reaching 590, and as many as 11017 patents.
RN-0191 is a small interfering RNA (siRNA) therapy being developed by Rona Therapeutics to target PCSK9, a protein that plays a critical role in regulating cholesterol levels. This innovative therapy aims to lower low-density lipoprotein cholesterol (LDL-C) in patients with hypercholesterolemia. It has completed Phase 1 clinical trials in both Australia and China and is preparing for Phase 2 clinical studies.