Moxonidine: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Moxonidine's R&D Progress
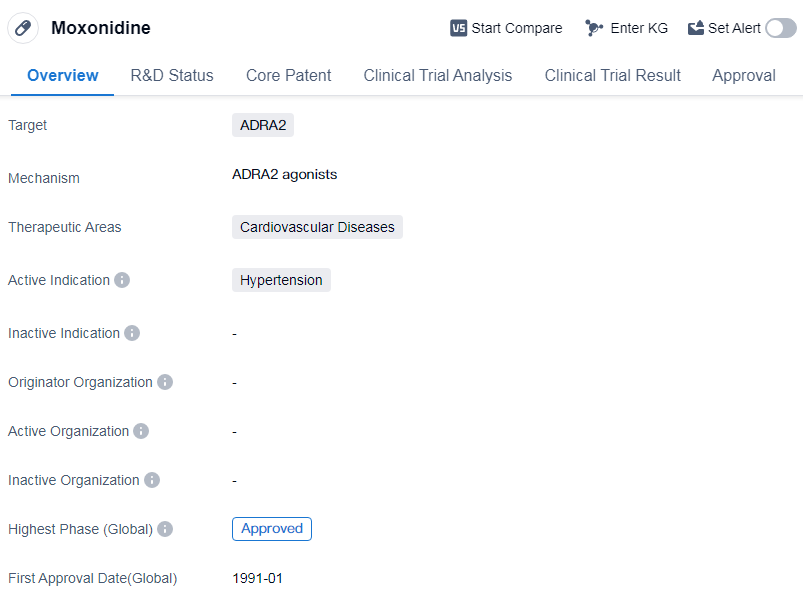
Moxonidine is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets ADRA2, making it suitable for the treatment of hypertension. Moxonidine was first approved in Germany in January 1991, marking its initial entry into the global market. Since then, it has gained approval in various other countries, establishing its credibility and effectiveness in treating hypertension. The drug's approval in China further highlights its global recognition and acceptance.
As a small molecule drug, Moxonidine is designed to interact with ADRA2 receptors, which are involved in regulating blood pressure. By targeting these receptors, the drug aims to reduce hypertension and manage cardiovascular diseases effectively. This mechanism of action makes Moxonidine a valuable option for patients suffering from high blood pressure.
The drug has been approved in global markets, with its highest phase being approved, which signifies its safety and efficacy profile. It has undergone rigorous testing and evaluation to meet the necessary regulatory standards, ensuring its suitability for patient use. The drug's long-standing presence in the market since its initial approval in 1991 further demonstrates its reliability and effectiveness.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Moxonidine: ADRA2 agonists
ADRA2 agonists are a class of drugs that act on the alpha-2 adrenergic receptors in the body. These receptors are found in various tissues, including the brain, blood vessels, and smooth muscles. ADRA2 agonists bind to these receptors and activate them, leading to specific physiological responses.
From a biomedical perspective, ADRA2 agonists are primarily used to regulate the release of norepinephrine, a neurotransmitter involved in the body's stress response. By activating the alpha-2 adrenergic receptors, these agonists can inhibit the release of norepinephrine, resulting in a decrease in sympathetic nervous system activity. This can lead to various effects, such as reduced blood pressure, sedation, and analgesia.
ADRA2 agonists have a wide range of medical applications. They are commonly used in the treatment of hypertension (high blood pressure) as they help relax blood vessels and reduce peripheral vascular resistance. These drugs can also be used as sedatives or anxiolytics to alleviate anxiety and promote relaxation. Additionally, ADRA2 agonists have been investigated for their potential role in managing pain, as they can modulate pain signals in the central nervous system.
It's important to note that the specific effects and applications of ADRA2 agonists may vary depending on the particular drug within this class. Different ADRA2 agonists may have varying selectivity for the alpha-2 adrenergic receptors and exhibit different pharmacokinetic properties. Therefore, the choice of ADRA2 agonist and its dosage will depend on the desired therapeutic outcome and individual patient characteristics.
Drug Target R&D Trends for Moxonidine
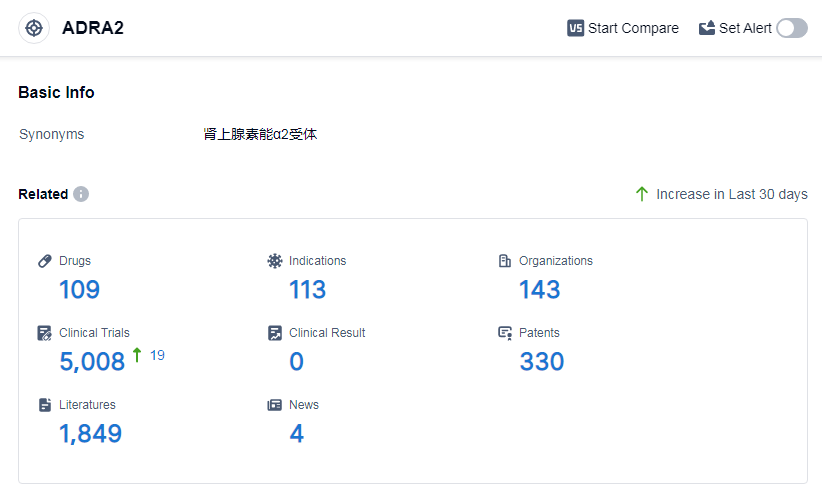
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 109 ADRA2 drugs worldwide, from 143 organizations, covering 113 indications, and conducting 5008 clinical trials.
The analysis of target ADRA2 from various perspectives provides valuable insights into the current competitive landscape and future development. Merck & Co., Inc., Senju Pharmaceutical Co., Ltd., and AbbVie, Inc. are the companies with the highest stage of development on this target. The approved indications for drugs targeting ADRA2 include hypertension, ocular hypertension, glaucoma, and depressive disorder.
Small molecule drugs dominate the drug type analysis, indicating intense competition and ongoing research and development efforts. China, the United States, and Japan are the countries with the highest progress under the current target, with China showing significant growth.
Overall, the analysis suggests a competitive landscape with multiple companies and countries actively involved in the development of drugs targeting ADRA2. The future development of this target holds potential for advancements in the treatment of various indications and increased market presence in China.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Moxonidine is a small molecule drug that targets ADRA2 receptors and is primarily used for the treatment of hypertension. It has gained approval in global markets, with its initial approval dating back to 1991 in Germany. The drug's approval in multiple countries highlights its credibility and effectiveness in managing cardiovascular diseases.