Deep Scientific Insights on granisetron hydrochloride's R&D Progress, Mechanism of Action, and Drug Target
Granisetron hydrochloride's R&D Progress
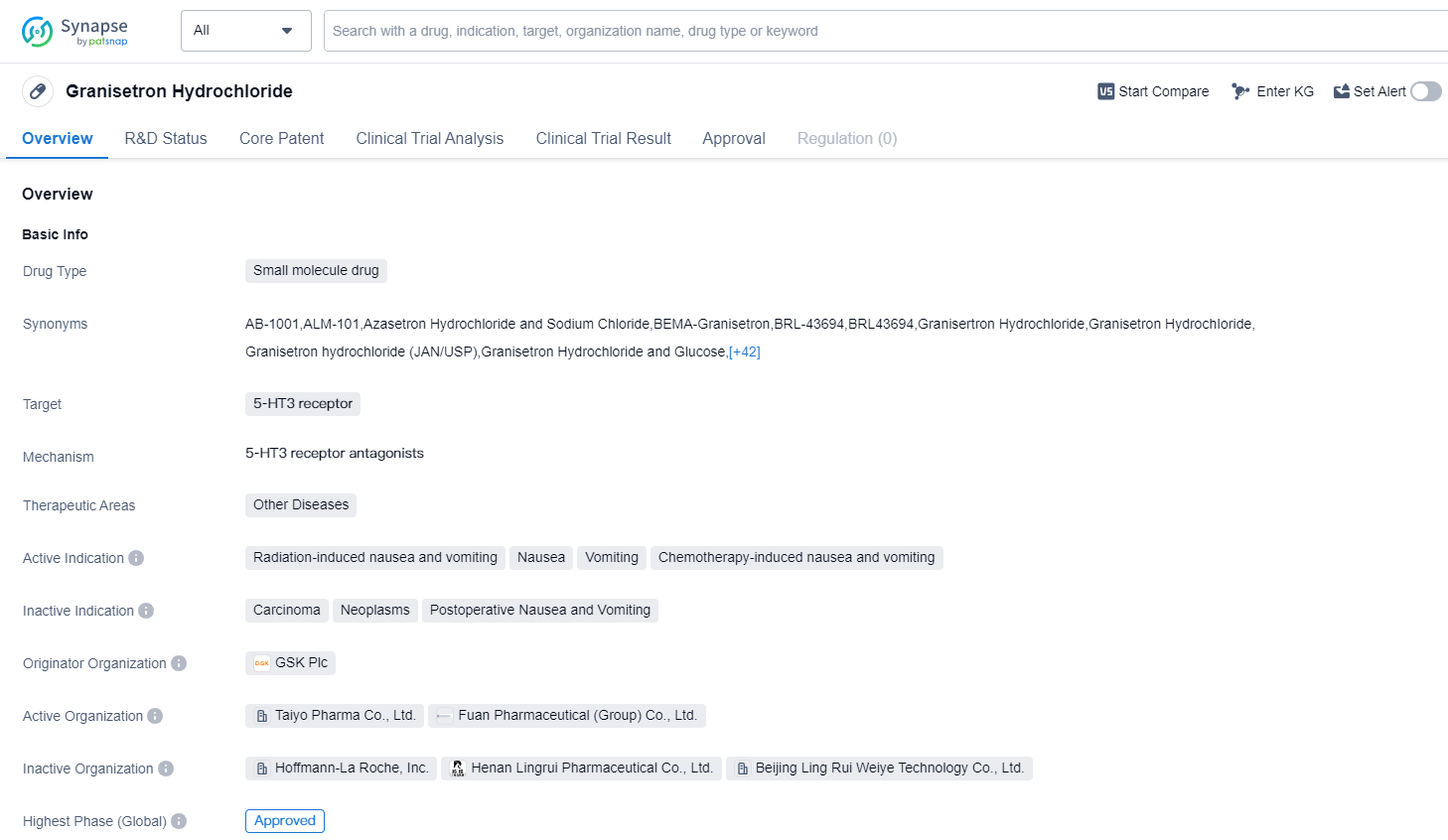
Granisetron Hydrochloride is a small molecule drug that targets the 5-HT3 receptor. It is primarily used in the treatment of various types of nausea and vomiting, including radiation-induced nausea and vomiting, nausea, vomiting, and chemotherapy-induced nausea and vomiting. The drug was first approved for use in the United States in December 1993.
Granisetron Hydrochloride is classified as a small molecule drug, which means it is composed of relatively low molecular weight compounds. It specifically targets the 5-HT3 receptor, which is involved in the regulation of nausea and vomiting. By blocking this receptor, Granisetron Hydrochloride helps to alleviate these symptoms in patients.
The drug is indicated for the treatment of radiation-induced nausea and vomiting, which can occur as a side effect of radiation therapy for cancer. It is also used to manage nausea and vomiting associated with other conditions, such as chemotherapy-induced nausea and vomiting. Additionally, Granisetron Hydrochloride is effective in treating general nausea and vomiting.
The originator organization of Granisetron Hydrochloride is GSK Plc, a multinational pharmaceutical company. The highest R&D phase of this drug is approved. The first approval of Granisetron Hydrochloride occurred in the United States in December 1993, making it available to patients in this country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for granisetron hydrochloride: 5-HT3 receptor
5-HT3 receptor antagonists are a class of drugs that act on the 5-HT3 receptors in the body. These receptors are located in the central and peripheral nervous systems and are involved in the transmission of signals related to nausea and vomiting.
From a biomedical perspective, 5-HT3 receptor antagonists are commonly used in the field of biomedicine to prevent and treat nausea and vomiting caused by various conditions such as chemotherapy-induced nausea, postoperative nausea, and vomiting, and radiation-induced nausea. They work by blocking the action of serotonin, a neurotransmitter that plays a role in triggering nausea and vomiting, from binding to the 5-HT3 receptors. This helps to reduce the sensation of nausea and prevent vomiting.
Some examples of 5-HT3 receptor antagonists include ondansetron, granisetron, and palonosetron. These drugs are often prescribed in clinical settings and are available in various formulations such as tablets, injections, and patches. They are typically used in combination with other medications to provide effective relief from nausea and vomiting.
Overall, 5-HT3 receptor antagonists are an important class of drugs in biomedicine that help manage and alleviate symptoms of nausea and vomiting in various medical conditions.
Drug Target R&D Trends for granisetron hydrochloride
The 5-HT3 receptor, also known as the serotonin receptor, plays a crucial role in the human body. It is primarily found in the central and peripheral nervous systems, particularly in the gastrointestinal tract and the brain. Activation of the 5-HT3 receptor by serotonin, a neurotransmitter, leads to various physiological responses such as regulating mood, appetite, and sleep. Additionally, this receptor is involved in the regulation of nausea and vomiting, making it a target for antiemetic drugs used in chemotherapy and postoperative settings. Understanding the role of the 5-HT3 receptor is essential for developing therapeutic interventions that can modulate its activity and potentially treat various disorders related to serotonin dysregulation.
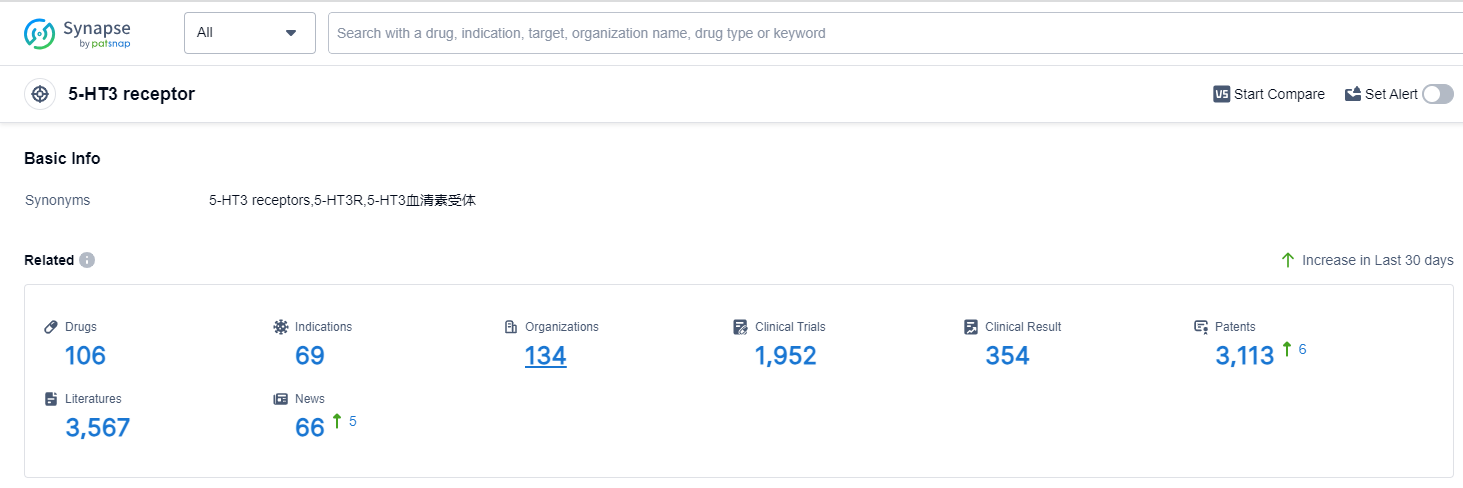
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 106 5-HT3 receptor drugs worldwide, from 134 organizations, covering 69 indications, and conducting 1952 clinical trials.
The analysis of the target 5-HT3 receptor reveals a competitive landscape with multiple companies contributing to its development. Fuan Pharmaceutical (Group) Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Helsinn Holding SA are the leading companies with the highest number of approved drugs. The indications with the most approved drugs are Nausea, Vomiting, and Chemotherapy-induced nausea and vomiting. Small molecule drugs are the most prominent drug type, indicating intense competition in the market. China, the United States, Japan, and the European Union are the countries/locations showing the fastest development, with China leading in terms of approved drugs. The future development of the target 5-HT3 receptor is promising, with ongoing research and development efforts by various companies and countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Granisetron Hydrochloride is a small molecule drug that targets the 5-HT3 receptor. It is primarily used to treat various types of nausea and vomiting, including radiation-induced nausea and vomiting, nausea, vomiting, and chemotherapy-induced nausea and vomiting. The drug was first approved in the United States in December 1993 and is manufactured by GSK Plc. Its approval in the global market highlights its effectiveness and widespread use in the treatment of these symptoms.