The Camrelizumab-Rivoeranib combo Demonstrates Survival Benefit in Unresectable Hepatocellular Carcinoma
Immunotherapy combining immune checkpoint inhibitors (ICIs) with anti-angiogenesis tyrosine kinase inhibitors (TKIs) has been shown to improve overall survival compared to anti-angiogenesis therapy alone in advanced solid tumors, but not in hepatocellular carcinoma.
Dynamic duo
Recently, a study has been published in The Lancet, which conducted a clinical trial comparing the efficacy and safety of the anti-PD-1 antibody camrelizumab combined with the VEGFR2-targeted TKI rivoceranib (also known as apatinib) versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma.
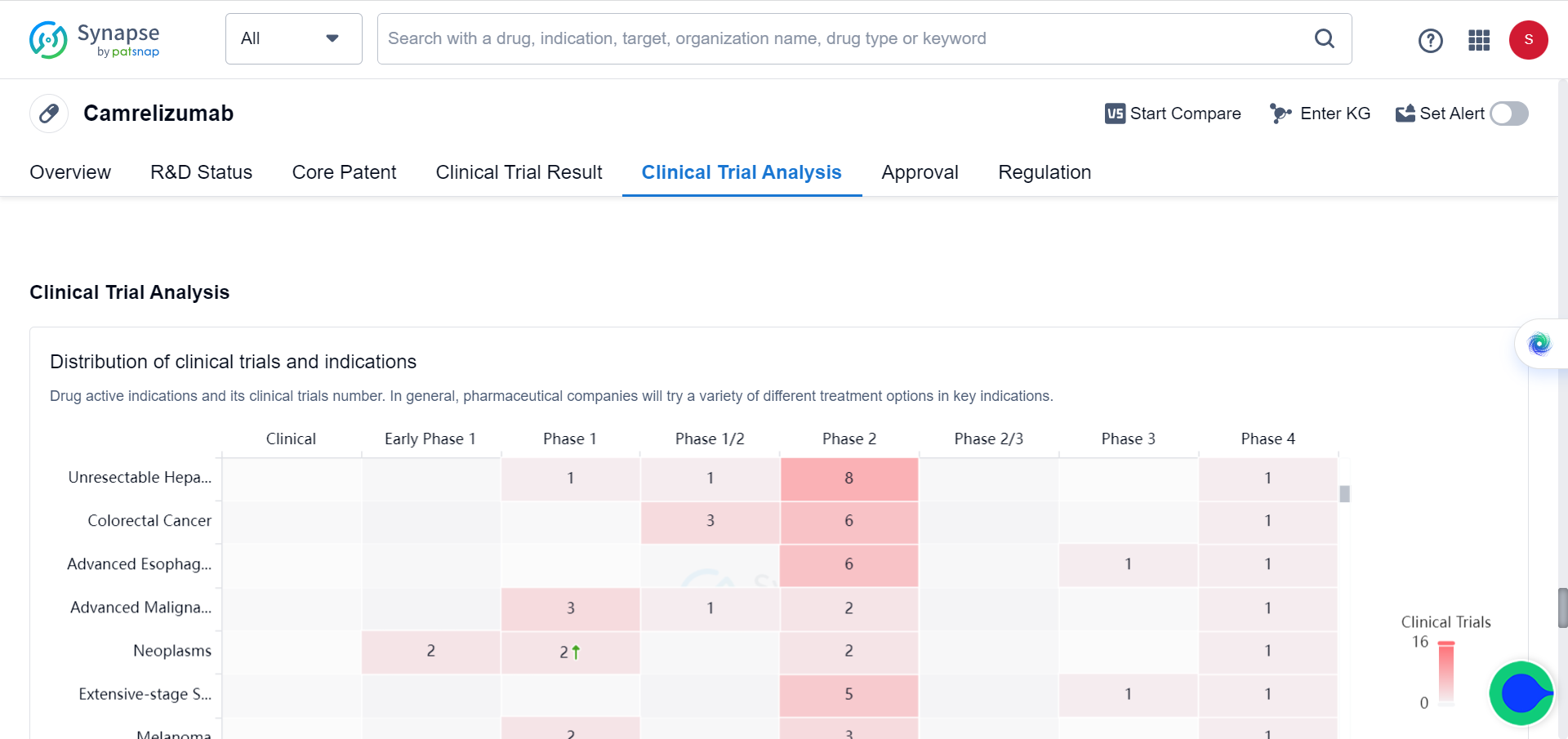
Between June 28, 2019 and March 24, 2021, 543 patients were randomly assigned to camrelizumab-rivoeranib (n=272) or sorafenib (n=271). In a preliminary analysis of progression-free survival with a median follow-up of 7.8 months (IQR 4.1-10.6), camrelizumab-rivoeranib significantly improved median progression-free survival compared to sorafenib (5.6 months [95% CI 5.5–6.3] vs 3.7 months [2.8–3.7]; hazard ratio [HR] 0.52 [95% CI 0.41–0.65]). In the overall survival analysis with a median follow-up of 14.5 months (IQR 9.1–18.7), median overall survival was significantly extended with camrelizumab-rivoeranib versus sorafenib. This study is registered as NCT03764293 and included in the Synapse database.
The most common grade 3 or 4 treatment-related adverse events were hypertension (102 [38%] of 272 patients in the camrelizumab-rivoceranib group vs 40 [15%] of 269 in the sorafenib group), palmar-plantar erythrodysesthesia syndrome (33 [12%] vs 41 [15%]), increased aspartate aminotransferase (45 [17%] vs 14 [5%]), and increased alanine aminotransferase (35 [13%] vs 8 [3%]).
Treatment-related serious adverse events were reported in 66 (24%) patients in the camremizumab-rivoceranib group and 16 (6%) in the sorafenib group. There were two treatment-related deaths: one patient (multi-organ dysfunction syndrome) in the camrelizumab-rivoceranib group and one patient (respiratory and circulatory failure) in the sorafenib group.
ICIs and Anti-Angiogenic Agents
Based on the results of the SHARP, SHARP-Asia-Pacific, and REFLECT trials, sorafenib and lenvatinib (TKIs) have been established as the standard first-line treatment for unresectable hepatocellular carcinoma. However, the improvement in median overall survival compared to placebo is only marginal. In recent years, immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 pathway have emerged as new treatment options for advanced hepatocellular carcinoma. But only a small proportion of patients respond to ICI monotherapy, and no substantial survival increase over sorafenib has been observed with first-line treatment.
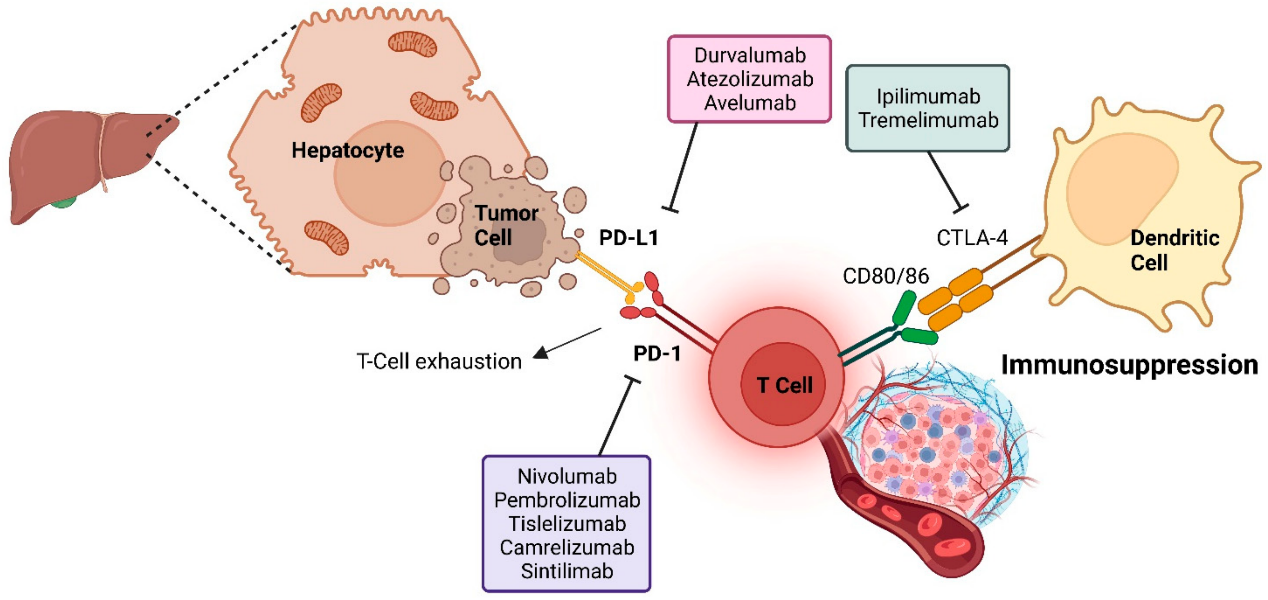
To improve the immunosuppressive tumor microenvironment and enhance tumor response to immunotherapy, some phase 3 trials have evaluated ICIs plus anti-angiogenic agents as the first-line treatment for unresectable hepatocellular carcinoma. The global IMbrave150, COSMIC-312, LEAP-002, and Chinese ORIENT-32 trials have assessed this approach, and the results of combination therapy have been mixed. Only ICI plus anti-VEGF antibody has significantly improved overall survival, while multi-kinase TKIs have not. Oral TKIs are gaining attention due to their convenient administration, enhancing dosing flexibility via their shorter half-life. They also potentially eliminate the need for gastroscopy, which is required for bevacizumab therapy for hepatocellular carcinoma.
Furthermore, in the phase 3 HIMALAYA trial, dual immunotherapy with tremelimumab plus durvalumab demonstrated superior overall survival over sorafenib. However, the median overall survival of all systemic therapies in phase 3 trials of unresectable hepatocellular carcinoma is less than 2 years. Therefore, there remains a medical need for additional effective first-line regimens.
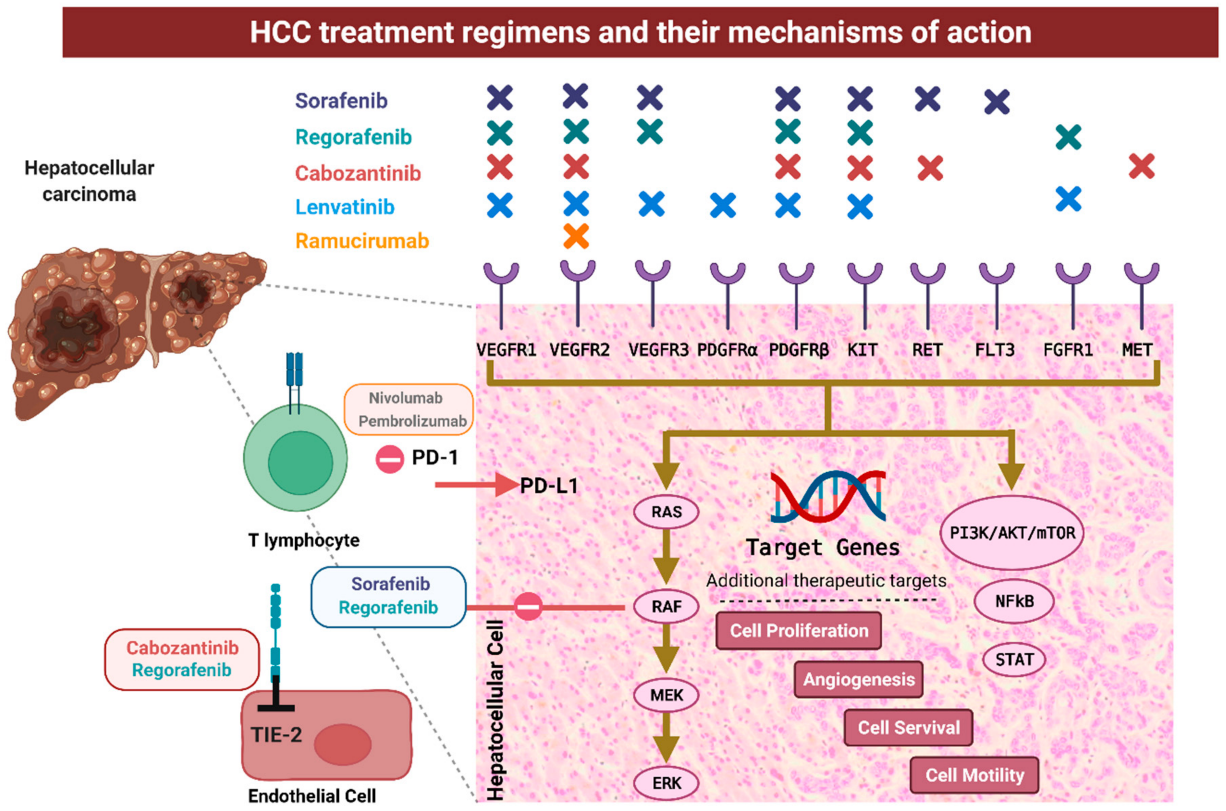
Rivoeranib is a small molecule, highly selective VEGFR2-targeting TKI that exerts antitumor activity by inhibiting tumor cell proliferation and angiogenesis as well as by counteracting the immunosuppressive effects of the tumor microenvironment. Both camrelizumab and rivoeranib have shown efficacy and safety in advanced hepatocellular carcinoma and are approved in China as second-line monotherapies. In a phase 1 study in pretreated hepatocellular carcinoma and gastric or gastroesophageal junction cancer, camrelizumab plus rivoeranib demonstrated encouraging antitumor activity and acceptable tolerability. Subsequently, in a phase 2 trial in advanced liver cancer, camrelizumab combined with rivoeranib as second-line therapy showed improved clinical efficacy compared to historical data with camrelizumab or rivoeranib monotherapy; additionally, the objective response rate (ORR) with first-line combination treatment was 34.3% (95% CI 23.3–46.6), median progression-free survival was 5.7 months (95% CI 5.4–7.4), and 18-month overall survival was 58.1%.
Significant improvement
This study is the first to report a phase 3 trial showing significant benefit in progression-free survival and overall survival with an anti-PD-1/PD-L1 antibody plus an oral small molecule TKI as first-line treatment for unresectable hepatocellular carcinoma. Camrelizumab-rivoeranib met the dual primary endpoints, demonstrating a 6.9-month improvement in median overall survival, a 1.9-month improvement in median progression-free survival (per BIRC RECIST 1.1) compared to sorafenib, with a 38% reduction in risk of death and 48% reduction in risk of progression or death. The survival benefits of combination therapy were supported by significantly higher response rates and more durable responses, as well as higher disease control rates compared to sorafenib.
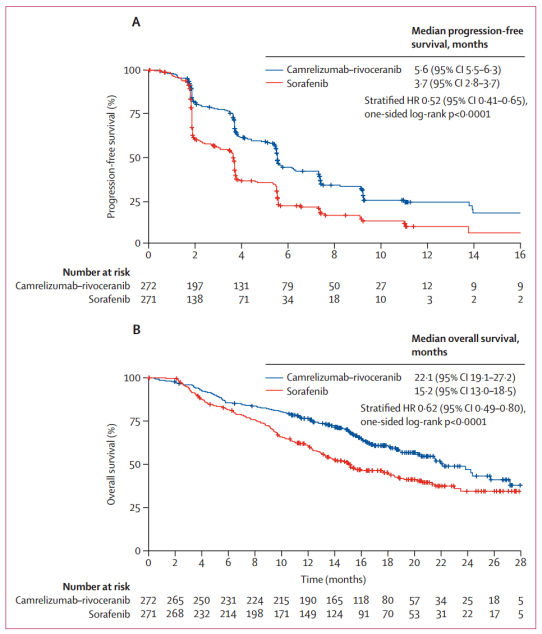
Tumor objective responses evaluated using BIRC per RECIST 1.1 were in line with those evaluated using BIRC per modified RECIST and by investigators per RECIST 1.1. The improvement in ORR for camrelizumab-rivoeranib versus sorafenib (25% vs. 6% per RECIST 1.1) was consistent with that reported for atezolizumab-bevacizumab in IMbrave150 (30% vs. 11%), and better than atezolizumab-cabozantinib in COSMIC-312 (11% vs. 4%). Among all patients treated with camrelizumab-rivoeranib, 78% achieved disease control, and 35% of patients assessed for target lesions after the baseline had a tumor reduction of ≥30% with the combination, indicating a broad potential for clinical benefit.
Camrelizumab plus rivoeranib demonstrated statistically significant and clinically meaningful improvements in progression-free survival and overall survival compared to sorafenib, with manageable safety. The overall favorable benefit-risk profile supports camrelizumab plus rivoeranib as a new first-line treatment option for previously untreated patients with unresectable hepatocellular carcinoma.

Reference
S. Qin, S. L Chan et al., Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study, The Lancet, 2023, (23)00961-3.