What are HSP90 inhibitors and how do you quickly get the latest development progress?
HSP90, or heat shock protein 90, is a crucial molecular chaperone found in the human body. It plays a vital role in maintaining protein homeostasis and ensuring proper folding, stability, and function of various client proteins. HSP90 is involved in numerous cellular processes, including signal transduction, cell cycle control, and protein degradation. It interacts with a wide range of client proteins, including oncogenic proteins, making it an attractive target for therapeutic intervention in cancer and other diseases. Understanding the role of HSP90 in the human body provides valuable insights for the development of novel pharmaceutical interventions and targeted therapies.
The HSP90 field is expected to be exciting in the next decade. Geldanamycin-based drugs are being rigorously evaluated for their clinical activity, while a series of synthetic small-molecule agents are entering preclinical and clinical development. Particular areas of interest include the potential for orally active HSP90 inhibitors and the development of isoform-selective drugs that target specific members of the HSP90 family, such as DMAG-N-OXIDE. HSP90 inhibitors may also be evaluated in diseases other than cancer, where protein folding defects are involved in the disease pathology. It is predicted that additional molecular chaperones will now be targeted for therapeutic intervention in cancer and other diseases. Furthermore, a portfolio of drugs can be envisaged that target various points in the protein quality control pathways of malignant cells and other disease states.
The mechanism of action of HSP90 inhibitors
HSP90 inhibitors are a type of drug that target and inhibit the activity of heat shock protein 90 (HSP90). Heat shock proteins are a group of proteins that play a crucial role in maintaining cellular homeostasis and protecting cells from stress. HSP90 is a specific type of heat shock protein that is involved in the folding, stabilization, and activation of various client proteins, including oncogenic proteins that promote cancer cell growth.
By inhibiting HSP90, HSP90 inhibitors disrupt the proper folding and function of client proteins, leading to their degradation and inhibition of their downstream signaling pathways. This can have therapeutic implications, particularly in cancer treatment, as many cancer cells rely on HSP90 for the stability and activity of oncogenic proteins that drive their growth and survival.
HSP90 inhibitors have shown promise as potential anticancer agents and are being investigated in preclinical and clinical studies. They have the potential to target multiple signaling pathways simultaneously, making them attractive candidates for combination therapies. However, their development and clinical use face challenges due to the complex nature of HSP90 and the potential for off-target effects.
In summary, HSP90 inhibitors are drugs that specifically target and inhibit the activity of HSP90, a heat shock protein involved in the folding and activation of various client proteins. They have potential therapeutic applications, particularly in cancer treatment, by disrupting oncogenic protein signaling pathways.
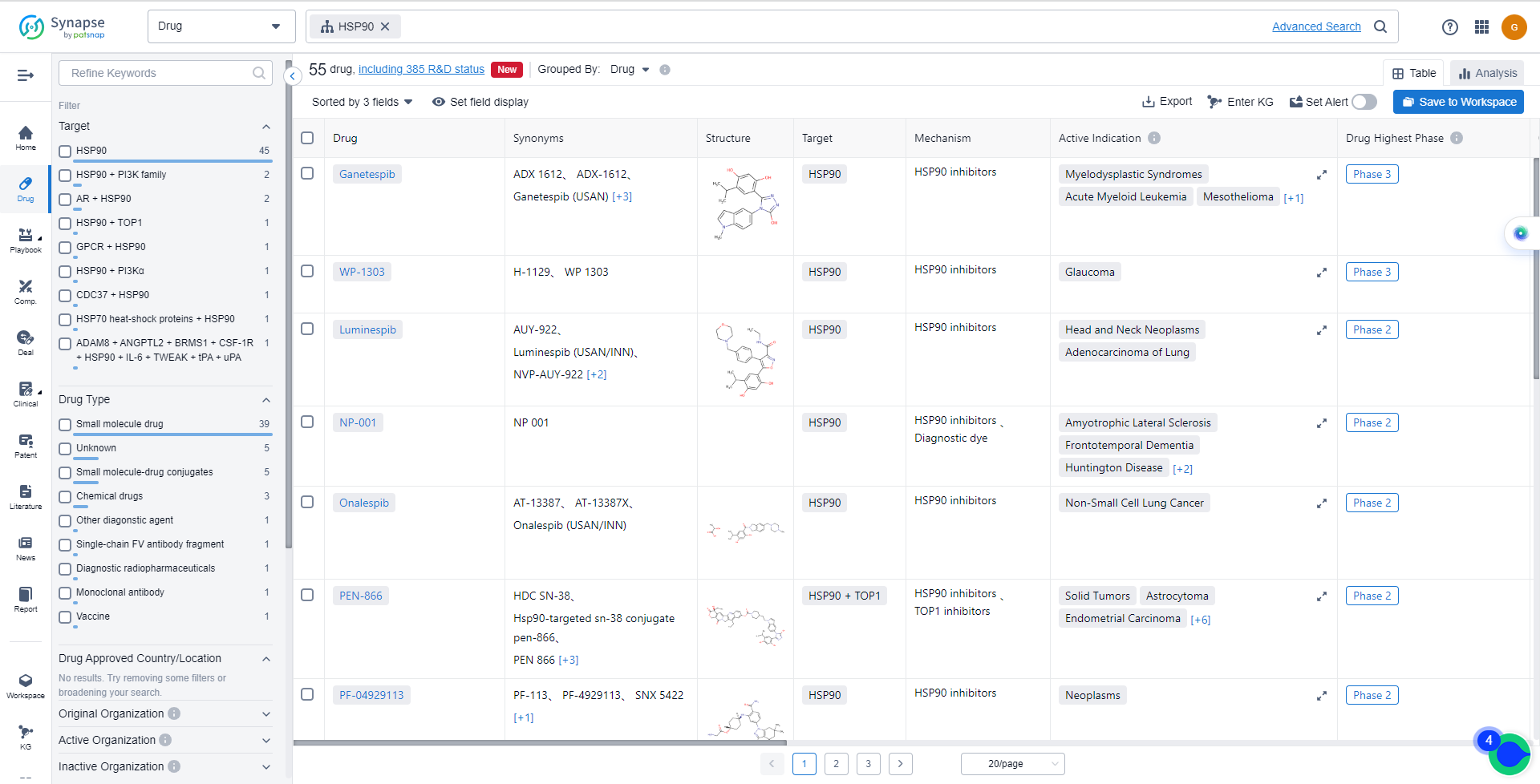
Catalog of HSP90 Inhibitors
The currently marketed HSP90 inhibitors include:
For more information, please click on the image below.
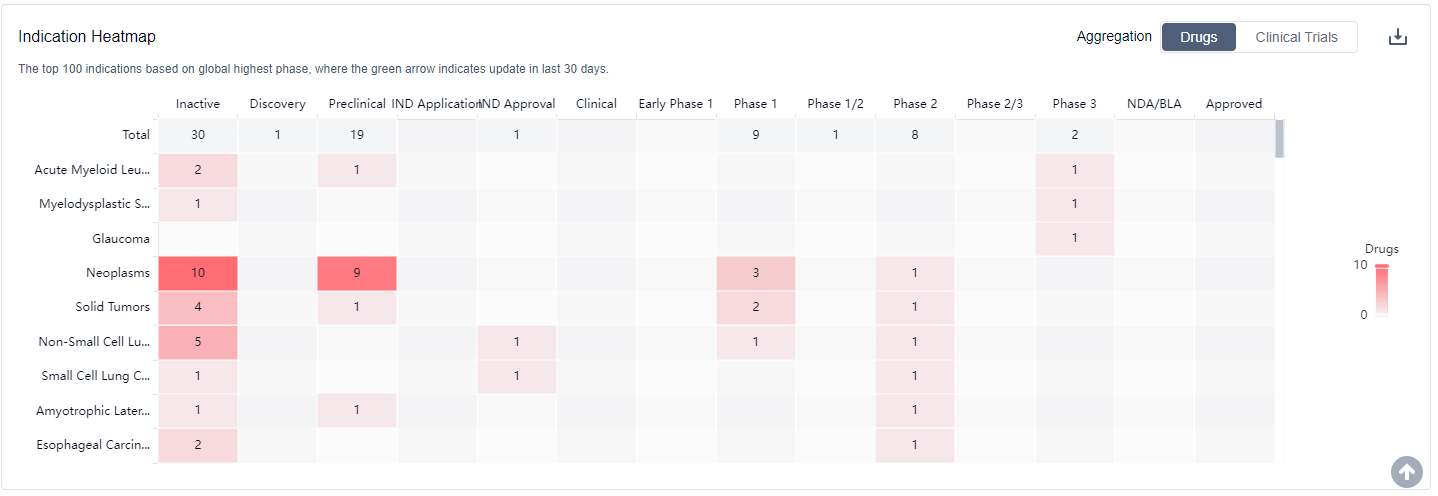
What is the purpose of using HSP90 inhibitors?
Clinical trials of Hsp90 inhibitors involve various types of cancer, with the most common indication being broad-spectrum tumors.For more information, please click on the image below to log in and search.
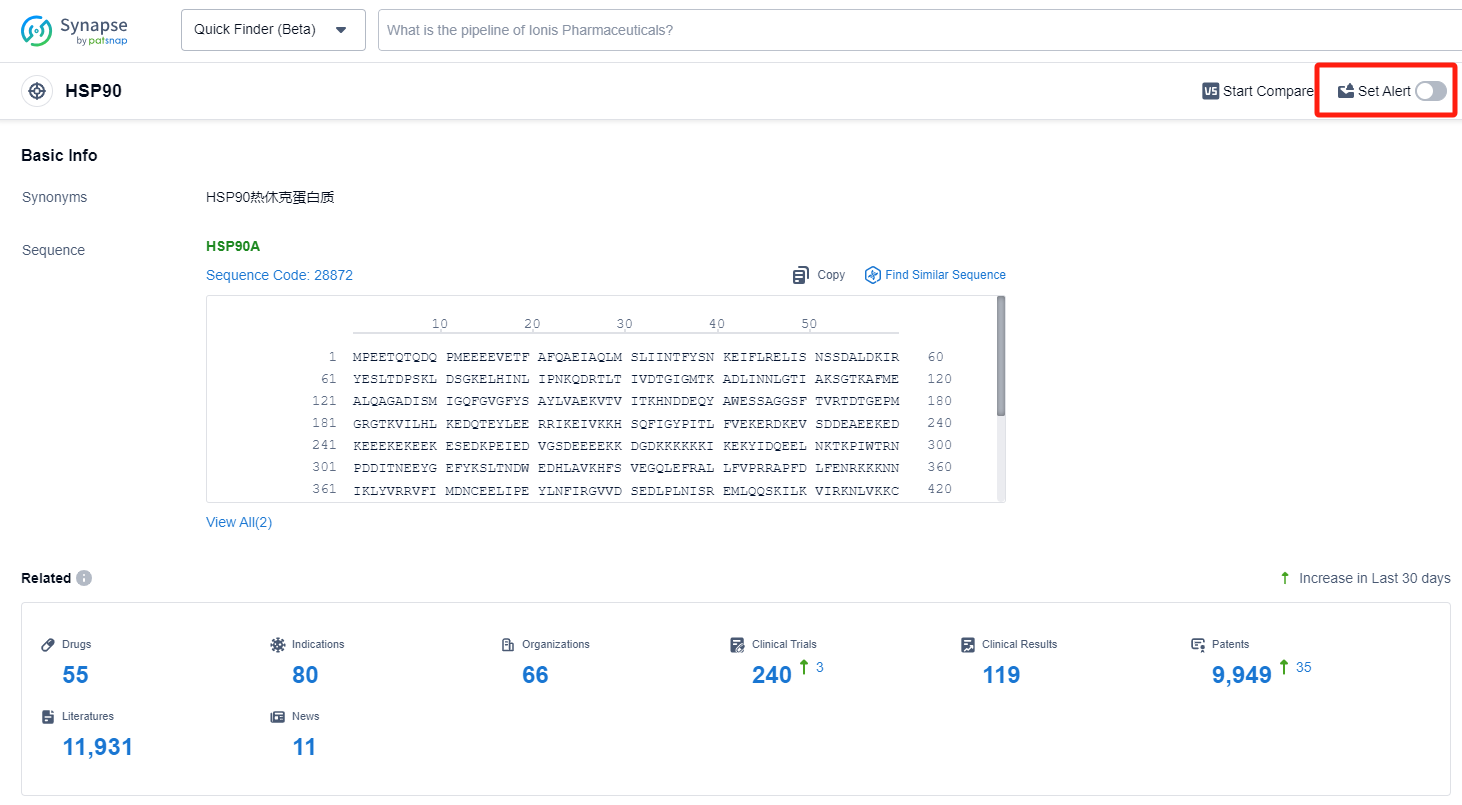
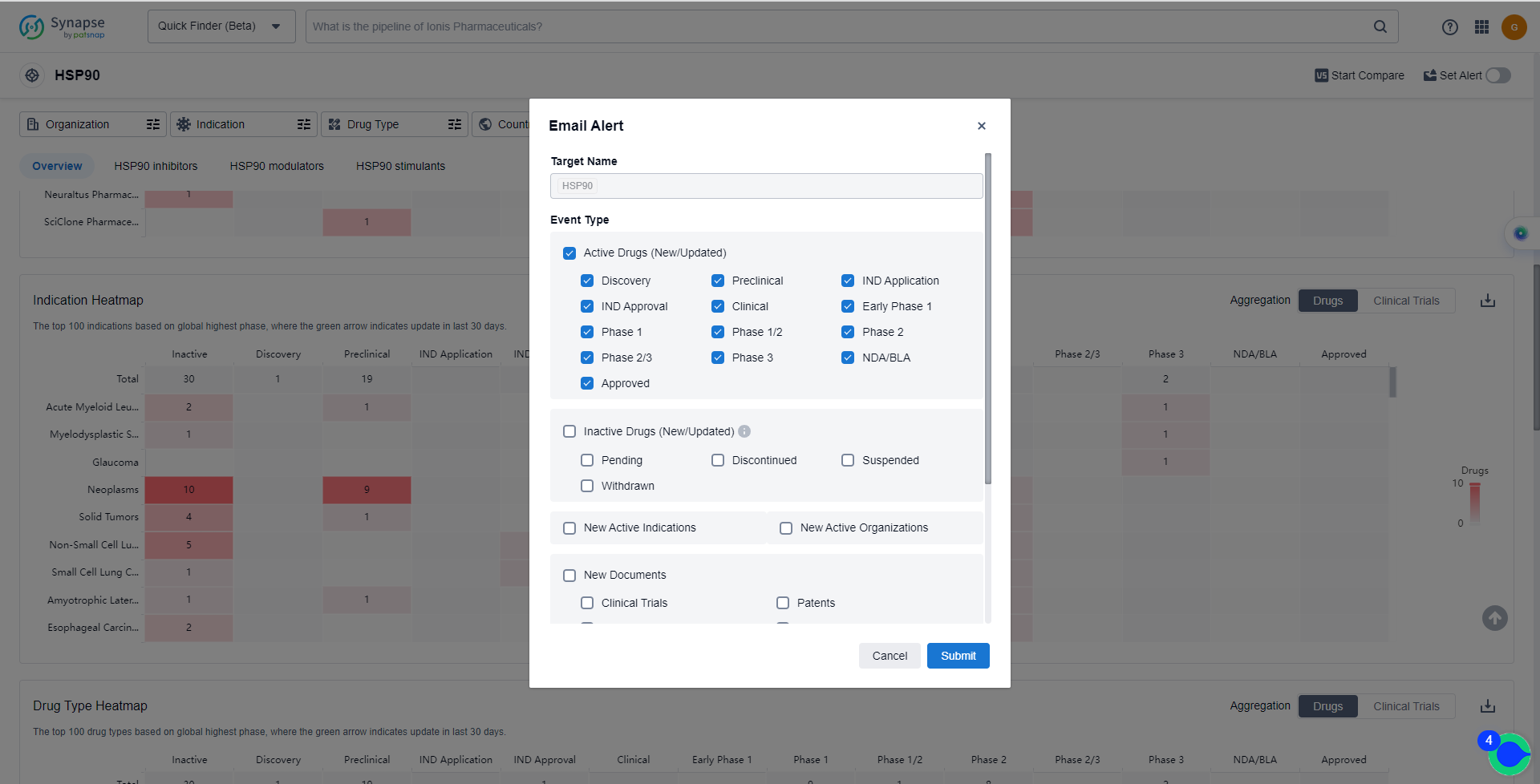
How can I get the most recent advancements in HSP90 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of HSP90 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!